| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Detected and Quantified |
|---|
| Creation Date | 2005-11-16 15:48:42 UTC |
|---|
| Update Date | 2021-09-14 14:57:20 UTC |
|---|
| HMDB ID | HMDB0000221 |
|---|
| Secondary Accession Numbers | - HMDB0000799
- HMDB0006341
- HMDB00221
- HMDB00799
- HMDB06341
|
|---|
| Metabolite Identification |
|---|
| Common Name | NADPH |
|---|
| Description | NADPH is the reduced form of NADP+, and NADP+ is the oxidized form of NADPH. Nicotinamide adenine dinucleotide phosphate (NADP) is a coenzyme composed of ribosylnicotinamide 5'-phosphate (NMN) coupled with a pyrophosphate linkage to 5'-phosphate adenosine 2',5'-bisphosphate. NADP serves as an electron carrier in a number of reactions, being alternately oxidized (NADP+) and reduced (NADPH). NADP is formed through the addition of a phosphate group to the 2' position of the adenosyl nucleotide through an ester linkage (Dorland, 27th ed). This extra phosphate is added by the enzyme NAD+ kinase and removed via NADP+ phosphatase. NADP is also known as TPN (triphosphopyridine nucleotide) and it is an important cofactor used in anabolic reactions in all forms of cellular life. Examples include the Calvin cycle, cholesterol synthesis, fatty acid elongation, and nucleic acid synthesis (Wikipedia ). |
|---|
| Structure | NC(=O)C1=CN(C=CC1)[C@@H]1O[C@H](COP(O)(=O)OP(O)(=O)OC[C@H]2O[C@H]([C@H](OP(O)(O)=O)[C@@H]2O)N2C=NC3=C2N=CN=C3N)[C@@H](O)[C@H]1O InChI=1S/C21H30N7O17P3/c22-17-12-19(25-7-24-17)28(8-26-12)21-16(44-46(33,34)35)14(30)11(43-21)6-41-48(38,39)45-47(36,37)40-5-10-13(29)15(31)20(42-10)27-3-1-2-9(4-27)18(23)32/h1,3-4,7-8,10-11,13-16,20-21,29-31H,2,5-6H2,(H2,23,32)(H,36,37)(H,38,39)(H2,22,24,25)(H2,33,34,35)/t10-,11-,13-,14-,15-,16-,20-,21-/m1/s1 |
|---|
| Synonyms | | Value | Source |
|---|
| Dihydronicotinamide-adenine dinucleotide phosphate | ChEBI | | NADPH dihydro-nicotinamide-adenine-dinucleotide phosphATE | ChEBI | | Reduced nicotinamide adenine dinucleotide phosphate | ChEBI | | Reduced nicotinamide-adenine dinucleotide phosphate | ChEBI | | TPNH | ChEBI | | Dihydronicotinamide-adenine dinucleotide phosphoric acid | Generator | | NADPH dihydro-nicotinamide-adenine-dinucleotide phosphoric acid | Generator | | Reduced nicotinamide adenine dinucleotide phosphoric acid | Generator | | Reduced nicotinamide-adenine dinucleotide phosphoric acid | Generator | | Nadph dihydro-nicotinamide-adenine-dinucleotidephosphoric acid | Generator | | Coenzyme II | MeSH | | Dinucleotide phosphate, nicotinamide-adenine | MeSH | | NADP | MeSH | | Nicotinamide adenine dinucleotide phosphate | MeSH | | Nicotinamide-adenine dinucleotide phosphate | MeSH | | Nucleotide, triphosphopyridine | MeSH | | Phosphate, nicotinamide-adenine dinucleotide | MeSH | | Triphosphopyridine nucleotide | MeSH | | NADPH | HMDB | | Nicotinamide-adenine dinucleotide phosphate, reduced | HMDB | | Reduced triphosphopyridine nucleotide | HMDB | | Triphosphopyridine nucleotide, reduced | HMDB | | beta-NADPH | HMDB | | beta-Nicotinamide-adenine-dinucleotide-phosphoric acid | HMDB | | beta-TPNH | HMDB | | β-NADPH | HMDB | | β-Nicotinamide-adenine-dinucleotide-phosphoric acid | HMDB | | β-TPNH | HMDB |
|
|---|
| Chemical Formula | C21H30N7O17P3 |
|---|
| Average Molecular Weight | 745.4209 |
|---|
| Monoisotopic Molecular Weight | 745.091102105 |
|---|
| IUPAC Name | {[(2R,3R,4R,5R)-2-(6-amino-9H-purin-9-yl)-5-[({[({[(2R,3S,4R,5R)-5-(3-carbamoyl-1,4-dihydropyridin-1-yl)-3,4-dihydroxyoxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy](hydroxy)phosphoryl}oxy)methyl]-4-hydroxyoxolan-3-yl]oxy}phosphonic acid |
|---|
| Traditional Name | nadph |
|---|
| CAS Registry Number | 53-57-6 |
|---|
| SMILES | NC(=O)C1=CN(C=CC1)[C@@H]1O[C@H](COP(O)(=O)OP(O)(=O)OC[C@H]2O[C@H]([C@H](OP(O)(O)=O)[C@@H]2O)N2C=NC3=C2N=CN=C3N)[C@@H](O)[C@H]1O |
|---|
| InChI Identifier | InChI=1S/C21H30N7O17P3/c22-17-12-19(25-7-24-17)28(8-26-12)21-16(44-46(33,34)35)14(30)11(43-21)6-41-48(38,39)45-47(36,37)40-5-10-13(29)15(31)20(42-10)27-3-1-2-9(4-27)18(23)32/h1,3-4,7-8,10-11,13-16,20-21,29-31H,2,5-6H2,(H2,23,32)(H,36,37)(H,38,39)(H2,22,24,25)(H2,33,34,35)/t10-,11-,13-,14-,15-,16-,20-,21-/m1/s1 |
|---|
| InChI Key | ACFIXJIJDZMPPO-NNYOXOHSSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as (5'->5')-dinucleotides. These are dinucleotides where the two bases are connected via a (5'->5')-phosphodiester linkage. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Nucleosides, nucleotides, and analogues |
|---|
| Class | (5'->5')-dinucleotides |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | (5'->5')-dinucleotides |
|---|
| Alternative Parents | |
|---|
| Substituents | - (5'->5')-dinucleotide
- Purine nucleotide sugar
- Purine ribonucleoside 2',5'-bisphosphate
- Purine ribonucleoside bisphosphate
- Purine ribonucleoside diphosphate
- Nicotinamide-nucleotide
- Pentose phosphate
- Pentose-5-phosphate
- N-glycosyl compound
- Glycosyl compound
- N-substituted nicotinamide
- Monosaccharide phosphate
- Organic pyrophosphate
- 6-aminopurine
- Imidazopyrimidine
- Dihydropyridinecarboxylic acid derivative
- Purine
- Dihydropyridine
- Monoalkyl phosphate
- Aminopyrimidine
- N-substituted imidazole
- Phosphoric acid ester
- Hydropyridine
- Alkyl phosphate
- Monosaccharide
- Organic phosphoric acid derivative
- Imidolactam
- Pyrimidine
- Azole
- Tetrahydrofuran
- Heteroaromatic compound
- Imidazole
- Secondary alcohol
- Oxacycle
- Azacycle
- Organoheterocyclic compound
- Carboximidic acid
- Carboximidic acid derivative
- Enamine
- Hydrocarbon derivative
- Organopnictogen compound
- Organic oxide
- Amine
- Organic oxygen compound
- Primary amine
- Alcohol
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Not Available | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Not Available |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Kovats Retention IndicesUnderivatized |
|---|
| Spectra |
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - NADPH GC-MS (TMS_1_1) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADPH GC-MS (TMS_1_2) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADPH GC-MS (TMS_1_3) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADPH GC-MS (TMS_1_4) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADPH GC-MS (TMS_1_5) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADPH GC-MS (TMS_1_6) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADPH GC-MS (TMS_1_7) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADPH GC-MS (TMS_1_8) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADPH GC-MS (TMS_2_1) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADPH GC-MS (TMS_2_2) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADPH GC-MS (TMS_2_3) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADPH GC-MS (TMS_2_4) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADPH GC-MS (TMS_2_5) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADPH GC-MS (TMS_2_6) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADPH GC-MS (TMS_2_7) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADPH GC-MS (TMS_2_8) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADPH GC-MS (TMS_2_9) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADPH GC-MS (TMS_2_10) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADPH GC-MS (TMS_2_11) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADPH GC-MS (TMS_2_12) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADPH GC-MS (TMS_2_13) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADPH GC-MS (TMS_2_14) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADPH GC-MS (TMS_2_15) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADPH GC-MS (TMS_2_16) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADPH GC-MS (TMS_2_17) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental LC-MS/MS | LC-MS/MS Spectrum - NADPH Quattro_QQQ 10V, Positive-QTOF (Annotated) | splash10-02tj-1838493720-b38b37650e8976905bb0 | 2019-11-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - NADPH Quattro_QQQ 25V, Positive-QTOF (Annotated) | splash10-0a4r-0841393100-6e4cbc8ac576e3e8b195 | 2019-11-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - NADPH Quattro_QQQ 40V, Positive-QTOF (Annotated) | splash10-0550-2694875730-0dc522520957880672cb | 2019-11-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - NADPH QTOF 15V, negative-QTOF | splash10-0006-0000000900-1448a5a4a9a9ec30bc1d | 2020-07-21 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - NADPH QTOF 17V, negative-QTOF | splash10-0006-0000000900-84c6720ae2b6d0d9ca35 | 2020-07-21 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - NADPH QTOF 20V, negative-QTOF | splash10-0006-0000100900-4ddda02ddc90d193fba1 | 2020-07-21 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - NADPH QTOF 23V, negative-QTOF | splash10-0006-0000201900-cbb70a91b1ac1fcb6e18 | 2020-07-21 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - NADPH QTOF 25V, negative-QTOF | splash10-0006-0001301900-2ec6727d0b32f09b91b6 | 2020-07-21 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - NADPH QTOF 27V, negative-QTOF | splash10-0006-0101502900-1d110f57242fe6b3d4eb | 2020-07-21 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - NADPH QTOF 30V, negative-QTOF | splash10-054o-1213903700-aa4ca72e492f0f0b7f75 | 2020-07-21 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - NADPH QTOF 33V, negative-QTOF | splash10-0a6u-1313903400-9a6e4df99ff5a68effea | 2020-07-21 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - NADPH QTOF 35V, negative-QTOF | splash10-0a6r-1324903300-069588f3537afa016ac8 | 2020-07-21 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - NADPH QTOF 40V, negative-QTOF | splash10-0a6r-3535903000-746e661fe41dfe08b6d1 | 2020-07-21 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - NADPH QTOF 45V, negative-QTOF | splash10-0a6r-5946701000-9407142d1340518483bf | 2020-07-21 | HMDB team, MONA | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - NADPH 10V, Positive-QTOF | splash10-000i-0921103300-a94dc77e4683c53f1e1c | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - NADPH 20V, Positive-QTOF | splash10-000i-0901000000-5fcc7bc2ff00254e837a | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - NADPH 40V, Positive-QTOF | splash10-000i-0920000000-91a94a0d16f7db6907e1 | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - NADPH 10V, Negative-QTOF | splash10-0036-6900111800-7f73a4ecf431645a13cf | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - NADPH 20V, Negative-QTOF | splash10-003r-3901000000-b9bf964190caf0afebde | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - NADPH 40V, Negative-QTOF | splash10-057i-6900000000-f6e201cd60ed9314f31a | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - NADPH 10V, Positive-QTOF | splash10-000i-0900000200-c30f8bbbadabff3b10f6 | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - NADPH 20V, Positive-QTOF | splash10-000i-0911003300-322bf2f4fc18dbf667d6 | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - NADPH 40V, Positive-QTOF | splash10-002r-1901300000-6129e75df61dcc44c45a | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - NADPH 10V, Negative-QTOF | splash10-0006-0000000900-b62da30b8e49b793af7d | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - NADPH 20V, Negative-QTOF | splash10-004i-8301205900-8cec7794d03fc25fae8b | 2021-09-22 | Wishart Lab | View Spectrum |
NMR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
|
|---|
| Biological Properties |
|---|
| Cellular Locations | - Cytoplasm (predicted from logP)
|
|---|
| Biospecimen Locations | |
|---|
| Tissue Locations | |
|---|
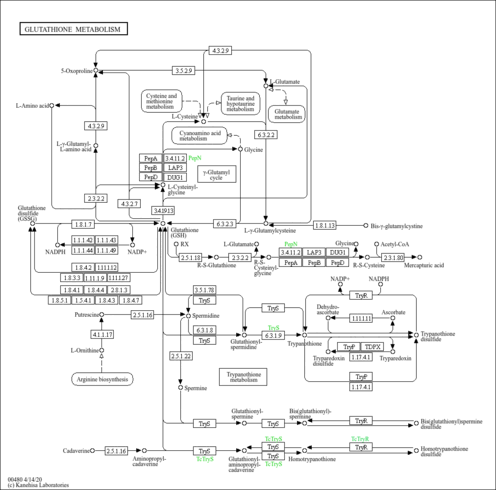
| Pathways | |
|---|
| Normal Concentrations |
|---|
| |
| Blood | Detected and Quantified | 51.0 (34.0-81.0) uM | Adult (>18 years old) | Both | Normal | | details |
|
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| Associated Disorders and Diseases |
|---|
| Disease References | None |
|---|
| Associated OMIM IDs | None |
|---|
| External Links |
|---|
| DrugBank ID | DB02338 |
|---|
| Phenol Explorer Compound ID | Not Available |
|---|
| FooDB ID | FDB021909 |
|---|
| KNApSAcK ID | C00019545 |
|---|
| Chemspider ID | 5673 |
|---|
| KEGG Compound ID | C00005 |
|---|
| BioCyc ID | NADPH |
|---|
| BiGG ID | Not Available |
|---|
| Wikipedia Link | Nicotinamide adenine dinucleotide phosphate |
|---|
| METLIN ID | 3691 |
|---|
| PubChem Compound | 5884 |
|---|
| PDB ID | Not Available |
|---|
| ChEBI ID | 16474 |
|---|
| Food Biomarker Ontology | Not Available |
|---|
| VMH ID | Not Available |
|---|
| MarkerDB ID | Not Available |
|---|
| Good Scents ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Seelbach, Karsten; Riebel, Bettina; Hummel, Werner; Kula, Maria-Regina; Tishkov, Vladimir I.; Egorov, Alexey M.; Wandrey, Christian; Kragl, Udo. A novel, efficient regenerating method of NADPH using a new formate dehydrogenase. Tetrahedron Letters (1996), 37(9), 1377-80. |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| General References | - Nakayama Y, Kinoshita A, Tomita M: Dynamic simulation of red blood cell metabolism and its application to the analysis of a pathological condition. Theor Biol Med Model. 2005 May 9;2:18. [PubMed:15882454 ]
- Shindo Y, Witt E, Han D, Epstein W, Packer L: Enzymic and non-enzymic antioxidants in epidermis and dermis of human skin. J Invest Dermatol. 1994 Jan;102(1):122-4. [PubMed:8288904 ]
- Iwata H, Tezuka Y, Kadota S, Hiratsuka A, Watabe T: Mechanism-based inactivation of human liver microsomal CYP3A4 by rutaecarpine and limonin from Evodia fruit extract. Drug Metab Pharmacokinet. 2005 Feb;20(1):34-45. [PubMed:15770073 ]
- Birkmayer GJ, Birkmayer W: Stimulation of endogenous L-dopa biosynthesis--a new principle for the therapy of Parkinson's disease. The clinical effect of nicotinamide adenine dinucleotide (NADH) and nicotinamide adenine dinucleotidephosphate (NADPH). Acta Neurol Scand Suppl. 1989;126:183-7. [PubMed:2618590 ]
- Lee AJ, Zhu BT: NADPH-dependent formation of polar and nonpolar estrogen metabolites following incubations of 17 beta-estradiol with human liver microsomes. Drug Metab Dispos. 2004 Aug;32(8):876-83. [PubMed:15258114 ]
- Kochansky CJ, Xia YQ, Wang S, Cato B, Creighton M, Vincent SH, Franklin RB, Reed JR: Species differences in the elimination of a peroxisome proliferator-activated receptor agonist highlighted by oxidative metabolism of its acyl glucuronide. Drug Metab Dispos. 2005 Dec;33(12):1894-904. Epub 2005 Sep 23. [PubMed:16183782 ]
- Karanam BV, Hop CE, Liu DQ, Wallace M, Dean D, Satoh H, Komuro M, Awano K, Vincent SH: In vitro metabolism of MK-0767 [(+/-)-5-[(2,4-dioxothiazolidin-5-yl)methyl]-2-methoxy-N-[[(4-trifluoromethyl) phenyl]methyl]benzamide], a peroxisome proliferator-activated receptor alpha/gamma agonist. I. Role of cytochrome P450, methyltransferases, flavin monooxygenases, and esterases. Drug Metab Dispos. 2004 Sep;32(9):1015-22. [PubMed:15319344 ]
- Soglia JR, Contillo LG, Kalgutkar AS, Zhao S, Hop CE, Boyd JG, Cole MJ: A semiquantitative method for the determination of reactive metabolite conjugate levels in vitro utilizing liquid chromatography-tandem mass spectrometry and novel quaternary ammonium glutathione analogues. Chem Res Toxicol. 2006 Mar;19(3):480-90. [PubMed:16544956 ]
- Afanas'ev IB, Suslova TB, Cheremisina ZP, Abramova NE, Korkina LG: Study of antioxidant properties of metal aspartates. Analyst. 1995 Mar;120(3):859-62. [PubMed:7741242 ]
- Lin CC, Wong BK, Burgey CS, Gibson CR, Singh R: In vitro metabolism of a thrombin inhibitor and quantitation of metabolically generated cyanide. J Pharm Biomed Anal. 2005 Oct 4;39(5):1014-20. Epub 2005 Jul 14. [PubMed:16023819 ]
- Conley AJ, Pattison JC, Bird IM: Variations in adrenal androgen production among (nonhuman) primates. Semin Reprod Med. 2004 Nov;22(4):311-26. [PubMed:15635499 ]
|
|---|