| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Detected and Quantified |
|---|
| Creation Date | 2005-11-16 15:48:42 UTC |
|---|
| Update Date | 2023-02-21 17:15:02 UTC |
|---|
| HMDB ID | HMDB0000660 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | D-Fructose |
|---|
| Description | Fructose, or levulose, is a levorotatory monosaccharide and an isomer of glucose (C6H12O6). Pure fructose has a sweet taste similar to cane sugar, but with a "fruity" aroma. Pure, dry fructose is a sweet, white, odorless, crystalline solid, and is the most water-soluble of all the sugars. Although fructose is a hexose (6-carbon sugar), it generally exists as a 5-member hemiketal ring (a furanose). This structure is responsible for the long metabolic pathway and high reactivity compared to glucose. Fructose is a reducing sugar, as are all monosaccharides. Fructose is found in many foods including honey, tree fruits, berries, melons, and some root vegetables, such as beets, sweet potatoes, parsnips, and onions. Commercially, fructose is derived from sugar cane, sugar beets, and maize. Fructose is also derived from the digestion of sucrose, a disaccharide consisting of glucose and fructose that is broken down by enzymes during digestion. Fructose is the sweetest naturally occurring sugar, estimated to be twice as sweet as sucrose. It is used as a preservative and an intravenous infusion in parenteral feeding. Excessive consumption of fructose (especially from sugar-sweetened beverages) may contribute to insulin resistance, obesity, elevated LDL cholesterol and triglycerides, leading to metabolic syndrome (PMID: 26429086 ). Fructose exists in foods either as a monosaccharide (free fructose) or as a unit of a disaccharide (sucrose). Free fructose is absorbed directly by the intestine. When fructose is consumed in the form of sucrose, it is digested (broken down) and then absorbed as free fructose. As sucrose comes into contact with the membrane of the small intestine, the enzyme sucrase catalyzes the cleavage of sucrose to yield one glucose unit and one fructose unit, which are then each absorbed. After absorption, it enters the hepatic portal vein and is directed toward the liver. fructose absorption occurs on the mucosal membrane via facilitated transport involving GLUT5 transport proteins. Since the concentration of fructose is higher in the lumen, fructose is able to flow down a concentration gradient into the enterocytes, assisted by transport proteins. Fructose may be transported out of the enterocyte across the basolateral membrane by either GLUT2 or GLUT5, although the GLUT2 transporter has a greater capacity for transporting fructose, and, therefore, the majority of fructose is transported out of the enterocyte through GLUT2. The catabolism of fructose is sometimes referred to as fructolysis. In fructolysis, the enzyme fructokinase produces fructose 1-phosphate, which is split by aldolase B to produce the trioses dihydroxyacetone phosphate (DHAP) and glyceraldehyde. Unlike glycolysis, in fructolysis the triose glyceraldehyde lacks a phosphate group. A third enzyme, triokinase, is therefore required to phosphorylate glyceraldehyde, producing glyceraldehyde 3-phosphate. The resulting trioses can enter the gluconeogenic pathway for glucose or glycogen synthesis, or be further catabolized through the lower glycolytic pathway to pyruvate. Fructose metabolism leads to significant increases of plasma uric acid levels (PMID: 28420204 ). In fructolysis, fructose 1-phosphate accumulates, and intracellular phosphate decreases. This decrease stimulates AMP deaminase (AMPD), which catalyzes the degradation of AMP to inosine monophosphate, increasing the rate of purine degradation (PMID: 28420204 ). The purine degradation produces uric acid and generates mitochondrial oxidants. Mitochondrial oxidative stress then induces aconitase inhibition in the Krebs cycle, with accumulation of citrate and stimulation of ATP citrate lyase and fatty acid synthase (PMID: 28420204 ). The result is de novo lipogenesis and hepatic fat accumulation. Physiologically, the increase in intracellular uric acid is followed by an acute rise in circulating levels of uric acid, which is likely due to its release from the liver. Fructose also stimulates uric acid synthesis from amino acid precursors such as glycine. Moreover, long-term fructose administration suppresses renal excretion of uric acid, resulting in elevated serum uric acid levels. | Read more...
|---|
| Structure | OC[C@H]1O[C@](O)(CO)[C@@H](O)[C@@H]1O InChI=1S/C6H12O6/c7-1-3-4(9)5(10)6(11,2-8)12-3/h3-5,7-11H,1-2H2/t3-,4-,5+,6-/m1/s1 |
|---|
| Synonyms | | Value | Source |
|---|
| beta-D-Arabino-hexulose | ChEBI | | beta-D-Fructose | ChEBI | | beta-Fruit sugar | ChEBI | | beta-Levulose | ChEBI | | FRUCTOSE | ChEBI | | b-D-Arabino-hexulose | Generator | | Β-D-arabino-hexulose | Generator | | b-D-Fructose | Generator | | Β-D-fructose | Generator | | b-Fruit sugar | Generator | | Β-fruit sugar | Generator | | b-Levulose | Generator | | Β-levulose | Generator | | beta-D-Fructofuranose | HMDB | | beta-delta-Arabino-hexulose | HMDB | | beta-delta-Fructofuranose | HMDB | | beta-delta-Fructose | HMDB | | D-(-)-Fructose | HMDB | | delta-(-)-Fructose | HMDB | | delta-Fructose | HMDB | | FRU | HMDB | | Fructon | HMDB | | Levulose | HMDB | | Braun brand OF fructose | HMDB | | Fleboplast levulosa | HMDB | | Fresenius kabi brand OF fructose | HMDB | | Instituto farmacologico brand OF fructose | HMDB | | Levulosa | HMDB | | Levulosa ife | HMDB | | Grifols brand OF fructose | HMDB | | Levulosa baxter | HMDB | | Levulosado braun | HMDB | | Ern brand OF fructose | HMDB | | Levulosa grifols | HMDB | | Levulosa mein | HMDB | | Levulosado vitulia | HMDB | | Apir levulosa | HMDB | | Baxter brand OF fructose | HMDB | | Bieffe brand OF fructose | HMDB | | Levulosa braun | HMDB | | Levulosa ibys | HMDB | | Levulosa, apir | HMDB | | Levulosa, fleboplast | HMDB | | Levulosado bieffe medit | HMDB | | Plast apyr levulosa mein | HMDB |
| Show more...
|---|
| Chemical Formula | C6H12O6 |
|---|
| Average Molecular Weight | 180.1559 |
|---|
| Monoisotopic Molecular Weight | 180.063388116 |
|---|
| IUPAC Name | (2R,3S,4S,5R)-2,5-bis(hydroxymethyl)oxolane-2,3,4-triol |
|---|
| Traditional Name | fructose |
|---|
| CAS Registry Number | 53188-23-1 |
|---|
| SMILES | OC[C@H]1O[C@](O)(CO)[C@@H](O)[C@@H]1O |
|---|
| InChI Identifier | InChI=1S/C6H12O6/c7-1-3-4(9)5(10)6(11,2-8)12-3/h3-5,7-11H,1-2H2/t3-,4-,5+,6-/m1/s1 |
|---|
| InChI Key | RFSUNEUAIZKAJO-ARQDHWQXSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as c-glycosyl compounds. These are glycoside in which a sugar group is bonded through one carbon to another group via a C-glycosidic bond. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | C-glycosyl compounds |
|---|
| Alternative Parents | |
|---|
| Substituents | - C-glycosyl compound
- Pentose monosaccharide
- Monosaccharide
- Tetrahydrofuran
- Secondary alcohol
- Hemiacetal
- Oxacycle
- Organoheterocyclic compound
- Polyol
- Hydrocarbon derivative
- Primary alcohol
- Alcohol
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Physiological effect | |
|---|
| Disposition | Biological locationSourceEndogenousExogenousFoodAnimal originFruitVegetableHerb and spiceNutGourdPulseCereal and cereal productTeaBeverageAquatic originEggConfectioneryMilk and milk productOther milk productFermented milk productFermented milkUnfermented milk- Milk (Other mammals) (FooDB: FOOD00690)
- Milk (Human) (FooDB: FOOD00666)
- Milk (Cow) (FooDB: FOOD00618)
- Cow milk, pasteurized, vitamin A + D added, 0% fat (FooDB: FOOD00889)
- Cow milk, pasteurized, vitamin A + D added, 1% fat (FooDB: FOOD00890)
- Cow milk, pasteurized, vitamin A + D added, 2% fat (FooDB: FOOD00891)
- Cow milk, pasteurized, vitamin D added, 3.25% fat (FooDB: FOOD00892)
Baking goodBaby foodSoyUnclassified food or beverageDishFat and oilCocoa and cocoa productCoffee and coffee productSnack |
|---|
| Process | |
|---|
| Role | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | 119 - 122 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 778 mg/mL at 20 °C | YALKOWSKY,SH & DANNENFELSER,RM (1992) | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Experimental Collision Cross Sections |
|---|
| Predicted Molecular Properties | | Show more...
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Kovats Retention IndicesUnderivatizedDerivatized| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| D-Fructose,1TMS,isomer #1 | C[Si](C)(C)OC[C@H]1O[C@](O)(CO)[C@@H](O)[C@@H]1O | 1703.6 | Semi standard non polar | 33892256 | | D-Fructose,1TMS,isomer #2 | C[Si](C)(C)O[C@]1(CO)O[C@H](CO)[C@@H](O)[C@@H]1O | 1765.2 | Semi standard non polar | 33892256 | | D-Fructose,1TMS,isomer #3 | C[Si](C)(C)OC[C@@]1(O)O[C@H](CO)[C@@H](O)[C@@H]1O | 1697.8 | Semi standard non polar | 33892256 | | D-Fructose,1TMS,isomer #4 | C[Si](C)(C)O[C@H]1[C@H](O)[C@@H](CO)O[C@]1(O)CO | 1741.5 | Semi standard non polar | 33892256 | | D-Fructose,1TMS,isomer #5 | C[Si](C)(C)O[C@H]1[C@H](O)[C@@](O)(CO)O[C@@H]1CO | 1710.7 | Semi standard non polar | 33892256 | | D-Fructose,2TMS,isomer #1 | C[Si](C)(C)OC[C@H]1O[C@@](CO)(O[Si](C)(C)C)[C@@H](O)[C@@H]1O | 1788.8 | Semi standard non polar | 33892256 | | D-Fructose,2TMS,isomer #10 | C[Si](C)(C)O[C@H]1[C@H](O[Si](C)(C)C)[C@@H](CO)O[C@]1(O)CO | 1748.4 | Semi standard non polar | 33892256 | | D-Fructose,2TMS,isomer #2 | C[Si](C)(C)OC[C@H]1O[C@](O)(CO[Si](C)(C)C)[C@@H](O)[C@@H]1O | 1761.0 | Semi standard non polar | 33892256 | | D-Fructose,2TMS,isomer #3 | C[Si](C)(C)OC[C@H]1O[C@](O)(CO)[C@@H](O[Si](C)(C)C)[C@@H]1O | 1769.2 | Semi standard non polar | 33892256 | | D-Fructose,2TMS,isomer #4 | C[Si](C)(C)OC[C@H]1O[C@](O)(CO)[C@@H](O)[C@@H]1O[Si](C)(C)C | 1742.2 | Semi standard non polar | 33892256 | | D-Fructose,2TMS,isomer #5 | C[Si](C)(C)OC[C@@]1(O[Si](C)(C)C)O[C@H](CO)[C@@H](O)[C@@H]1O | 1777.4 | Semi standard non polar | 33892256 | | D-Fructose,2TMS,isomer #6 | C[Si](C)(C)O[C@H]1[C@H](O)[C@](CO)(O[Si](C)(C)C)O[C@@H]1CO | 1779.5 | Semi standard non polar | 33892256 | | D-Fructose,2TMS,isomer #7 | C[Si](C)(C)O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[Si](C)(C)C | 1783.9 | Semi standard non polar | 33892256 | | D-Fructose,2TMS,isomer #8 | C[Si](C)(C)OC[C@@]1(O)O[C@H](CO)[C@@H](O[Si](C)(C)C)[C@@H]1O | 1755.2 | Semi standard non polar | 33892256 | | D-Fructose,2TMS,isomer #9 | C[Si](C)(C)OC[C@@]1(O)O[C@H](CO)[C@@H](O)[C@@H]1O[Si](C)(C)C | 1766.2 | Semi standard non polar | 33892256 | | D-Fructose,3TMS,isomer #1 | C[Si](C)(C)OC[C@H]1O[C@@](CO[Si](C)(C)C)(O[Si](C)(C)C)[C@@H](O)[C@@H]1O | 1819.7 | Semi standard non polar | 33892256 | | D-Fructose,3TMS,isomer #10 | C[Si](C)(C)OC[C@@]1(O)O[C@H](CO)[C@@H](O[Si](C)(C)C)[C@@H]1O[Si](C)(C)C | 1778.1 | Semi standard non polar | 33892256 | | D-Fructose,3TMS,isomer #2 | C[Si](C)(C)OC[C@H]1O[C@@](CO)(O[Si](C)(C)C)[C@@H](O[Si](C)(C)C)[C@@H]1O | 1818.0 | Semi standard non polar | 33892256 | | D-Fructose,3TMS,isomer #3 | C[Si](C)(C)OC[C@H]1O[C@@](CO)(O[Si](C)(C)C)[C@@H](O)[C@@H]1O[Si](C)(C)C | 1813.7 | Semi standard non polar | 33892256 | | D-Fructose,3TMS,isomer #4 | C[Si](C)(C)OC[C@H]1O[C@](O)(CO[Si](C)(C)C)[C@@H](O[Si](C)(C)C)[C@@H]1O | 1801.5 | Semi standard non polar | 33892256 | | D-Fructose,3TMS,isomer #5 | C[Si](C)(C)OC[C@H]1O[C@](O)(CO[Si](C)(C)C)[C@@H](O)[C@@H]1O[Si](C)(C)C | 1788.5 | Semi standard non polar | 33892256 | | D-Fructose,3TMS,isomer #6 | C[Si](C)(C)OC[C@H]1O[C@](O)(CO)[C@@H](O[Si](C)(C)C)[C@@H]1O[Si](C)(C)C | 1770.4 | Semi standard non polar | 33892256 | | D-Fructose,3TMS,isomer #7 | C[Si](C)(C)OC[C@@]1(O[Si](C)(C)C)O[C@H](CO)[C@@H](O[Si](C)(C)C)[C@@H]1O | 1819.8 | Semi standard non polar | 33892256 | | D-Fructose,3TMS,isomer #8 | C[Si](C)(C)OC[C@@]1(O[Si](C)(C)C)O[C@H](CO)[C@@H](O)[C@@H]1O[Si](C)(C)C | 1802.3 | Semi standard non polar | 33892256 | | D-Fructose,3TMS,isomer #9 | C[Si](C)(C)O[C@H]1[C@H](O[Si](C)(C)C)[C@@H](CO)O[C@@]1(CO)O[Si](C)(C)C | 1790.2 | Semi standard non polar | 33892256 | | D-Fructose,4TMS,isomer #1 | C[Si](C)(C)OC[C@H]1O[C@@](CO[Si](C)(C)C)(O[Si](C)(C)C)[C@@H](O[Si](C)(C)C)[C@@H]1O | 1794.5 | Semi standard non polar | 33892256 | | D-Fructose,4TMS,isomer #2 | C[Si](C)(C)OC[C@H]1O[C@@](CO[Si](C)(C)C)(O[Si](C)(C)C)[C@@H](O)[C@@H]1O[Si](C)(C)C | 1787.6 | Semi standard non polar | 33892256 | | D-Fructose,4TMS,isomer #3 | C[Si](C)(C)OC[C@H]1O[C@@](CO)(O[Si](C)(C)C)[C@@H](O[Si](C)(C)C)[C@@H]1O[Si](C)(C)C | 1778.8 | Semi standard non polar | 33892256 | | D-Fructose,4TMS,isomer #4 | C[Si](C)(C)OC[C@H]1O[C@](O)(CO[Si](C)(C)C)[C@@H](O[Si](C)(C)C)[C@@H]1O[Si](C)(C)C | 1765.8 | Semi standard non polar | 33892256 | | D-Fructose,4TMS,isomer #5 | C[Si](C)(C)OC[C@@]1(O[Si](C)(C)C)O[C@H](CO)[C@@H](O[Si](C)(C)C)[C@@H]1O[Si](C)(C)C | 1784.1 | Semi standard non polar | 33892256 | | D-Fructose,5TMS,isomer #1 | C[Si](C)(C)OC[C@H]1O[C@@](CO[Si](C)(C)C)(O[Si](C)(C)C)[C@@H](O[Si](C)(C)C)[C@@H]1O[Si](C)(C)C | 1838.5 | Semi standard non polar | 33892256 | | D-Fructose,1TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC[C@H]1O[C@](O)(CO)[C@@H](O)[C@@H]1O | 1933.1 | Semi standard non polar | 33892256 | | D-Fructose,1TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)O[C@]1(CO)O[C@H](CO)[C@@H](O)[C@@H]1O | 1988.6 | Semi standard non polar | 33892256 | | D-Fructose,1TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OC[C@@]1(O)O[C@H](CO)[C@@H](O)[C@@H]1O | 1939.3 | Semi standard non polar | 33892256 | | D-Fructose,1TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)O[C@H]1[C@H](O)[C@@H](CO)O[C@]1(O)CO | 1955.3 | Semi standard non polar | 33892256 | | D-Fructose,1TBDMS,isomer #5 | CC(C)(C)[Si](C)(C)O[C@H]1[C@H](O)[C@@](O)(CO)O[C@@H]1CO | 1940.4 | Semi standard non polar | 33892256 | | D-Fructose,2TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC[C@H]1O[C@@](CO)(O[Si](C)(C)C(C)(C)C)[C@@H](O)[C@@H]1O | 2228.2 | Semi standard non polar | 33892256 | | D-Fructose,2TBDMS,isomer #10 | CC(C)(C)[Si](C)(C)O[C@H]1[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](CO)O[C@]1(O)CO | 2210.8 | Semi standard non polar | 33892256 | | D-Fructose,2TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)OC[C@H]1O[C@](O)(CO[Si](C)(C)C(C)(C)C)[C@@H](O)[C@@H]1O | 2201.7 | Semi standard non polar | 33892256 | | D-Fructose,2TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OC[C@H]1O[C@](O)(CO)[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O | 2213.8 | Semi standard non polar | 33892256 | | D-Fructose,2TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)OC[C@H]1O[C@](O)(CO)[C@@H](O)[C@@H]1O[Si](C)(C)C(C)(C)C | 2189.9 | Semi standard non polar | 33892256 | | D-Fructose,2TBDMS,isomer #5 | CC(C)(C)[Si](C)(C)OC[C@@]1(O[Si](C)(C)C(C)(C)C)O[C@H](CO)[C@@H](O)[C@@H]1O | 2218.9 | Semi standard non polar | 33892256 | | D-Fructose,2TBDMS,isomer #6 | CC(C)(C)[Si](C)(C)O[C@H]1[C@H](O)[C@](CO)(O[Si](C)(C)C(C)(C)C)O[C@@H]1CO | 2231.2 | Semi standard non polar | 33892256 | | D-Fructose,2TBDMS,isomer #7 | CC(C)(C)[Si](C)(C)O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[Si](C)(C)C(C)(C)C | 2232.1 | Semi standard non polar | 33892256 | | D-Fructose,2TBDMS,isomer #8 | CC(C)(C)[Si](C)(C)OC[C@@]1(O)O[C@H](CO)[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O | 2207.5 | Semi standard non polar | 33892256 | | D-Fructose,2TBDMS,isomer #9 | CC(C)(C)[Si](C)(C)OC[C@@]1(O)O[C@H](CO)[C@@H](O)[C@@H]1O[Si](C)(C)C(C)(C)C | 2214.3 | Semi standard non polar | 33892256 | | D-Fructose,3TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC[C@H]1O[C@@](CO[Si](C)(C)C(C)(C)C)(O[Si](C)(C)C(C)(C)C)[C@@H](O)[C@@H]1O | 2473.4 | Semi standard non polar | 33892256 | | D-Fructose,3TBDMS,isomer #10 | CC(C)(C)[Si](C)(C)OC[C@@]1(O)O[C@H](CO)[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O[Si](C)(C)C(C)(C)C | 2480.0 | Semi standard non polar | 33892256 | | D-Fructose,3TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)OC[C@H]1O[C@@](CO)(O[Si](C)(C)C(C)(C)C)[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O | 2489.7 | Semi standard non polar | 33892256 | | D-Fructose,3TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OC[C@H]1O[C@@](CO)(O[Si](C)(C)C(C)(C)C)[C@@H](O)[C@@H]1O[Si](C)(C)C(C)(C)C | 2491.3 | Semi standard non polar | 33892256 | | D-Fructose,3TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)OC[C@H]1O[C@](O)(CO[Si](C)(C)C(C)(C)C)[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O | 2480.8 | Semi standard non polar | 33892256 | | D-Fructose,3TBDMS,isomer #5 | CC(C)(C)[Si](C)(C)OC[C@H]1O[C@](O)(CO[Si](C)(C)C(C)(C)C)[C@@H](O)[C@@H]1O[Si](C)(C)C(C)(C)C | 2466.9 | Semi standard non polar | 33892256 | | D-Fructose,3TBDMS,isomer #6 | CC(C)(C)[Si](C)(C)OC[C@H]1O[C@](O)(CO)[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O[Si](C)(C)C(C)(C)C | 2471.4 | Semi standard non polar | 33892256 | | D-Fructose,3TBDMS,isomer #7 | CC(C)(C)[Si](C)(C)OC[C@@]1(O[Si](C)(C)C(C)(C)C)O[C@H](CO)[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O | 2485.5 | Semi standard non polar | 33892256 | | D-Fructose,3TBDMS,isomer #8 | CC(C)(C)[Si](C)(C)OC[C@@]1(O[Si](C)(C)C(C)(C)C)O[C@H](CO)[C@@H](O)[C@@H]1O[Si](C)(C)C(C)(C)C | 2479.7 | Semi standard non polar | 33892256 | | D-Fructose,3TBDMS,isomer #9 | CC(C)(C)[Si](C)(C)O[C@H]1[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](CO)O[C@@]1(CO)O[Si](C)(C)C(C)(C)C | 2481.2 | Semi standard non polar | 33892256 | | D-Fructose,4TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC[C@H]1O[C@@](CO[Si](C)(C)C(C)(C)C)(O[Si](C)(C)C(C)(C)C)[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O | 2718.5 | Semi standard non polar | 33892256 | | D-Fructose,4TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)OC[C@H]1O[C@@](CO[Si](C)(C)C(C)(C)C)(O[Si](C)(C)C(C)(C)C)[C@@H](O)[C@@H]1O[Si](C)(C)C(C)(C)C | 2731.6 | Semi standard non polar | 33892256 | | D-Fructose,4TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OC[C@H]1O[C@@](CO)(O[Si](C)(C)C(C)(C)C)[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O[Si](C)(C)C(C)(C)C | 2715.2 | Semi standard non polar | 33892256 | | D-Fructose,4TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)OC[C@H]1O[C@](O)(CO[Si](C)(C)C(C)(C)C)[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O[Si](C)(C)C(C)(C)C | 2713.5 | Semi standard non polar | 33892256 | | D-Fructose,4TBDMS,isomer #5 | CC(C)(C)[Si](C)(C)OC[C@@]1(O[Si](C)(C)C(C)(C)C)O[C@H](CO)[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O[Si](C)(C)C(C)(C)C | 2708.9 | Semi standard non polar | 33892256 | | D-Fructose,5TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC[C@H]1O[C@@](CO[Si](C)(C)C(C)(C)C)(O[Si](C)(C)C(C)(C)C)[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O[Si](C)(C)C(C)(C)C | 2919.5 | Semi standard non polar | 33892256 |
| Show more...
|---|
| Spectra |
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - D-Fructose GC-MS (Non-derivatized) - 70eV, Positive | splash10-076r-9600000000-f2c06850d0c3db9ec103 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - D-Fructose GC-MS (5 TMS) - 70eV, Positive | splash10-0fb9-5614950000-6ef14e948d063026f7ee | 2017-10-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - D-Fructose GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - D-Fructose GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - D-Fructose GC-MS (TMS_1_1) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - D-Fructose GC-MS (TMS_1_2) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - D-Fructose GC-MS (TMS_1_3) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - D-Fructose GC-MS (TMS_1_4) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - D-Fructose GC-MS (TMS_1_5) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - D-Fructose GC-MS (TMS_2_1) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - D-Fructose GC-MS (TMS_2_2) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - D-Fructose GC-MS (TMS_2_3) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - D-Fructose GC-MS (TMS_2_4) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - D-Fructose GC-MS (TMS_2_5) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - D-Fructose GC-MS (TMS_2_6) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - D-Fructose GC-MS (TMS_2_7) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - D-Fructose GC-MS (TMS_2_8) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - D-Fructose GC-MS (TMS_2_9) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - D-Fructose GC-MS (TMS_2_10) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - D-Fructose GC-MS (TMS_3_1) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - D-Fructose GC-MS (TMS_3_2) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - D-Fructose GC-MS (TMS_3_3) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - D-Fructose GC-MS (TMS_3_4) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - D-Fructose GC-MS (TMS_3_5) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - D-Fructose GC-MS (TMS_3_6) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental LC-MS/MS | LC-MS/MS Spectrum - D-Fructose Quattro_QQQ 10V, Positive-QTOF (Annotated) | splash10-0002-0900000000-2ce9036c30158be73d60 | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - D-Fructose Quattro_QQQ 25V, Positive-QTOF (Annotated) | splash10-00ri-9600000000-a826b29724713036f579 | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - D-Fructose Quattro_QQQ 40V, Positive-QTOF (Annotated) | splash10-007a-9200000000-7621d8d96132cc999f4c | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - D-Fructose LC-ESI-QTOF 10V, negative-QTOF | splash10-000i-9000000000-f40ae4502b6a2e3ac882 | 2020-07-21 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - D-Fructose LC-ESI-QTOF 20V, negative-QTOF | splash10-00di-9000000000-a6de82c4270a43535ef4 | 2020-07-21 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - D-Fructose LC-ESI-QTOF 40V, negative-QTOF | splash10-00di-9000000000-f9fa2c446096028a62b8 | 2020-07-21 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - D-Fructose 10V, Negative-QTOF | splash10-000i-9000000000-f40ae4502b6a2e3ac882 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - D-Fructose 20V, Negative-QTOF | splash10-00di-9000000000-a6de82c4270a43535ef4 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - D-Fructose 40V, Negative-QTOF | splash10-00di-9000000000-f9fa2c446096028a62b8 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - D-Fructose 10V, Positive-QTOF | splash10-001i-0900000000-2afc98a319bf6041adf0 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - D-Fructose 20V, Positive-QTOF | splash10-03ea-3900000000-1dd0cdea93f529e6ccb2 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - D-Fructose 40V, Positive-QTOF | splash10-0007-9100000000-d0f08ad6c9dee008067a | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - D-Fructose 10V, Negative-QTOF | splash10-004i-2900000000-74b62c8ea3678afcff8a | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - D-Fructose 20V, Negative-QTOF | splash10-01ta-1900000000-5a0f3f01ddf8c11c6370 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - D-Fructose 40V, Negative-QTOF | splash10-0006-9000000000-93c2b00aa44481aa8630 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - D-Fructose 10V, Negative-QTOF | splash10-056r-9800000000-b033c19e1db6b17bf7cf | 2021-09-23 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - D-Fructose 20V, Negative-QTOF | splash10-0a4i-9500000000-01306ef5e3c5a271dfb2 | 2021-09-23 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - D-Fructose 40V, Negative-QTOF | splash10-0a4l-9000000000-4e87bfc49b1664133053 | 2021-09-23 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - D-Fructose 10V, Positive-QTOF | splash10-001i-0900000000-6aefe19f3e63c1b3fbef | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - D-Fructose 20V, Positive-QTOF | splash10-03gm-9500000000-d2f178dbda765758ef3d | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - D-Fructose 40V, Positive-QTOF | splash10-0btd-9000000000-62631cc26542b523e7d7 | 2021-09-24 | Wishart Lab | View Spectrum |
NMR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Experimental 1D NMR | 1H NMR Spectrum (1D, 500 MHz, H2O, experimental) | 2012-12-04 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Experimental 1D NMR | 13C NMR Spectrum (1D, 400 MHz, H2O, experimental) | 2021-10-10 | Wishart Lab | View Spectrum | | Experimental 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, H2O, experimental) | 2012-12-05 | Wishart Lab | View Spectrum |
IR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M-H]-) | 2023-02-03 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M+H]+) | 2023-02-03 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M+Na]+) | 2023-02-03 | FELIX lab | View Spectrum |
| Show more...
|---|
| Biological Properties |
|---|
| Cellular Locations | |
|---|
| Biospecimen Locations | - Blood
- Cerebrospinal Fluid (CSF)
- Feces
- Saliva
- Urine
|
|---|
| Tissue Locations | - Liver
- Placenta
- Prostate
- Semen
|
|---|
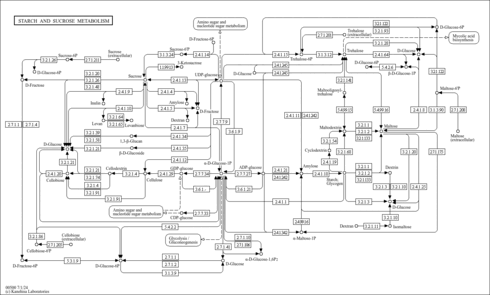
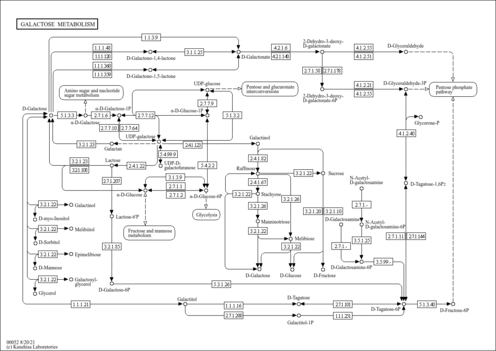
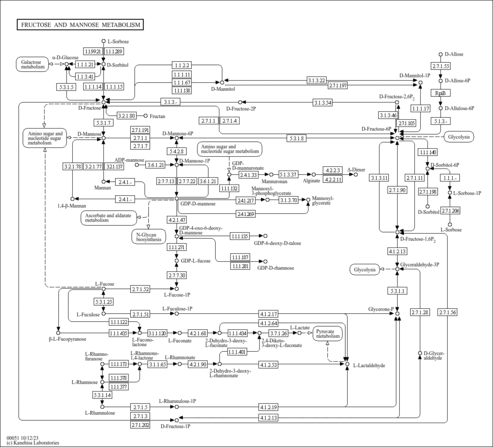
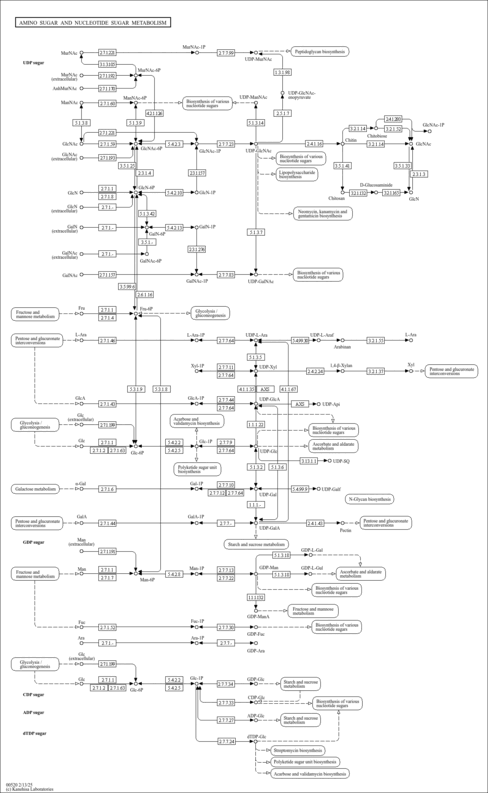
| Pathways | |
|---|
| Normal Concentrations |
|---|
| |
| Blood | Detected and Quantified | 31.0 +/- 3.0 uM | Adult (>18 years old) | Both | Normal | | details | | Blood | Detected and Quantified | 48.0 +/- 16.0 uM | Adult (>18 years old) | Male | Normal | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 240.0 (220.0 -260.0) uM | Adult (>18 years old) | Both | Normal | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 160 +/- 91 uM | Adult (>18 years old) | Both | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Not Specified | Not Specified | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Infant (0-1 year old) | Both | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Urine | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Urine | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Urine | Detected and Quantified | 129.518 +/- 61.708 umol/mmol creatinine | Children (1 - 13 years old) | Not Specified | Normal | | details | | Urine | Detected and Quantified | 70.38 +/- 107.54 umol/mmol creatinine | Infant (0-1 year old) | Not Specified | Normal | | details |
| Show more...
|---|
| Abnormal Concentrations |
|---|
| |
| Blood | Detected and Quantified | 12.00 +/- 3.8 uM | Adult (>18 years old) | Both | Diabetes | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Colorectal cancer | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Colorectal cancer | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Colorectal Cancer | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Colorectal cancer | | details | | Urine | Detected and Quantified | 8.4 +/- 7.0 umol/mmol creatinine | Adult (>18 years old) | Both | Diabetes | | details | | Urine | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Bladder cancer | | details | | Urine | Detected and Quantified | 94.126 +/- 154.083 umol/mmol creatinine | Children (1 - 13 years old) | Not Specified | Eosinophilic esophagitis | | details |
| Show more...
|---|
| Associated Disorders and Diseases |
|---|
| Disease References | | Colorectal cancer |
|---|
- Phua LC, Chue XP, Koh PK, Cheah PY, Ho HK, Chan EC: Non-invasive fecal metabonomic detection of colorectal cancer. Cancer Biol Ther. 2014 Apr;15(4):389-97. doi: 10.4161/cbt.27625. Epub 2014 Jan 14. [PubMed:24424155 ]
- Brown DG, Rao S, Weir TL, O'Malia J, Bazan M, Brown RJ, Ryan EP: Metabolomics and metabolic pathway networks from human colorectal cancers, adjacent mucosa, and stool. Cancer Metab. 2016 Jun 6;4:11. doi: 10.1186/s40170-016-0151-y. eCollection 2016. [PubMed:27275383 ]
- Sinha R, Ahn J, Sampson JN, Shi J, Yu G, Xiong X, Hayes RB, Goedert JJ: Fecal Microbiota, Fecal Metabolome, and Colorectal Cancer Interrelations. PLoS One. 2016 Mar 25;11(3):e0152126. doi: 10.1371/journal.pone.0152126. eCollection 2016. [PubMed:27015276 ]
- Goedert JJ, Sampson JN, Moore SC, Xiao Q, Xiong X, Hayes RB, Ahn J, Shi J, Sinha R: Fecal metabolomics: assay performance and association with colorectal cancer. Carcinogenesis. 2014 Sep;35(9):2089-96. doi: 10.1093/carcin/bgu131. Epub 2014 Jul 18. [PubMed:25037050 ]
| | Diabetes mellitus type 2 |
|---|
- Kawasaki T, Akanuma H, Yamanouchi T: Increased fructose concentrations in blood and urine in patients with diabetes. Diabetes Care. 2002 Feb;25(2):353-7. [PubMed:11815509 ]
| | Eosinophilic esophagitis |
|---|
- Slae, M., Huynh, H., Wishart, D.S. (2014). Analysis of 30 normal pediatric urine samples via NMR spectroscopy (unpublished work). NA.
|
|
|---|
| Associated OMIM IDs | - 114500 (Colorectal cancer)
- 125853 (Diabetes mellitus type 2)
- 610247 (Eosinophilic esophagitis)
|
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| Phenol Explorer Compound ID | Not Available |
|---|
| FooDB ID | FDB031286 |
|---|
| KNApSAcK ID | C00001117 |
|---|
| Chemspider ID | 388775 |
|---|
| KEGG Compound ID | C02336 |
|---|
| BioCyc ID | BETA-D-FRUCTOSE |
|---|
| BiGG ID | 33835 |
|---|
| Wikipedia Link | Fructose |
|---|
| METLIN ID | 135 |
|---|
| PubChem Compound | 439709 |
|---|
| PDB ID | FRU |
|---|
| ChEBI ID | 28645 |
|---|
| Food Biomarker Ontology | Not Available |
|---|
| VMH ID | FRU |
|---|
| MarkerDB ID | MDB00000210 |
|---|
| Good Scents ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Liu, Hong; Han, Dong; Meng, Xiang-bao; Li, Zhong-jun. Improved synthesis of fructose-derived 1,3,4-oxadiazole as novel antitumor agents. Journal of Chinese Pharmaceutical Sciences (2005), 14(4), 209-212. |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| General References | - Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, Laxman B, Mehra R, Lonigro RJ, Li Y, Nyati MK, Ahsan A, Kalyana-Sundaram S, Han B, Cao X, Byun J, Omenn GS, Ghosh D, Pennathur S, Alexander DC, Berger A, Shuster JR, Wei JT, Varambally S, Beecher C, Chinnaiyan AM: Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009 Feb 12;457(7231):910-4. doi: 10.1038/nature07762. [PubMed:19212411 ]
- Vicari E, La Vignera S, Castiglione R, Calogero AE: Sperm parameter abnormalities, low seminal fructose and reactive oxygen species overproduction do not discriminate patients with unilateral or bilateral post-infectious inflammatory prostato-vesiculo-epididymitis. J Endocrinol Invest. 2006 Jan;29(1):18-25. [PubMed:16553029 ]
- Bar A: Characteristics and significance of D-tagatose-induced liver enlargement in rats: An interpretative review. Regul Toxicol Pharmacol. 1999 Apr;29(2 Pt 2):S83-93. [PubMed:10341166 ]
- Andrade-Rocha FT: Semen analysis in an infertile man with seminal vesicles cysts associated with ipsilateral renal agenesis. Int Urol Nephrol. 2006;38(1):101-3. [PubMed:16502061 ]
- Andrade-Rocha FT: Seminal fructose levels in male infertility: relationship with sperm characteristics. Int Urol Nephrol. 1999;31(1):107-11. [PubMed:10408311 ]
- Gonzales GF, Villena A: True corrected seminal fructose level: a better marker of the function of seminal vesicles in infertile men. Int J Androl. 2001 Oct;24(5):255-60. [PubMed:11554981 ]
- Buemann B, Gesmar H, Astrup A, Quistorff B: Effects of oral D-tagatose, a stereoisomer of D-fructose, on liver metabolism in man as examined by 31P-magnetic resonance spectroscopy. Metabolism. 2000 Oct;49(10):1335-9. [PubMed:11079825 ]
- Koca Y, Ozdal OL, Celik M, Unal S, Balaban N: Antioxidant activity of seminal plasma in fertile and infertile men. Arch Androl. 2003 Sep-Oct;49(5):355-9. [PubMed:12893512 ]
- Williams AC, Ford WC: The role of glucose in supporting motility and capacitation in human spermatozoa. J Androl. 2001 Jul-Aug;22(4):680-95. [PubMed:11451366 ]
- Elshenawy S, Pinney SE, Stuart T, Doulias PT, Zura G, Parry S, Elovitz MA, Bennett MJ, Bansal A, Strauss JF 3rd, Ischiropoulos H, Simmons RA: The Metabolomic Signature of the Placenta in Spontaneous Preterm Birth. Int J Mol Sci. 2020 Feb 4;21(3). pii: ijms21031043. doi: 10.3390/ijms21031043. [PubMed:32033212 ]
- Malik VS, Hu FB: Fructose and Cardiometabolic Health: What the Evidence From Sugar-Sweetened Beverages Tells Us. J Am Coll Cardiol. 2015 Oct 6;66(14):1615-1624. doi: 10.1016/j.jacc.2015.08.025. [PubMed:26429086 ]
- Caliceti C, Calabria D, Roda A, Cicero AFG: Fructose Intake, Serum Uric Acid, and Cardiometabolic Disorders: A Critical Review. Nutrients. 2017 Apr 18;9(4). pii: nu9040395. doi: 10.3390/nu9040395. [PubMed:28420204 ]
| Show more...
|---|