| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Detected and Quantified |
|---|
| Creation Date | 2005-11-16 15:48:42 UTC |
|---|
| Update Date | 2023-05-30 20:55:58 UTC |
|---|
| HMDB ID | HMDB0000267 |
|---|
| Secondary Accession Numbers | - HMDB0000805
- HMDB00267
- HMDB0060262
- HMDB00805
- HMDB60262
| Show more accession numbers
|---|
| Metabolite Identification |
|---|
| Common Name | Pyroglutamic acid |
|---|
| Description | Pyroglutamic acid (5-oxoproline) is a cyclized derivative of L-glutamic acid. It is an uncommon amino acid derivative in which the free amino group of glutamic acid cyclizes to form a lactam. It is formed nonenzymatically from glutamate, glutamine, and gamma-glutamylated peptides, but it can also be produced by the action of gamma-glutamylcyclotransferase on an L-amino acid. Elevated blood levels may be associated with problems of glutamine or glutathione metabolism. This compound is found in substantial amounts in brain tissue and other tissues in bound form, especially skin. It is also present in plant tissues. It is sold, over the counter, as a "smart drug" for improving blood circulation in the brain. Pyroglutamate in the urine is a biomarker for the consumption of cheese. When present in sufficiently high levels, pyroglutamic acid can act as an acidogen and a metabotoxin. An acidogen is an acidic compound that induces acidosis, which has multiple adverse effects on many organ systems. A metabotoxin is an endogenously produced metabolite that causes adverse health effects at chronically high levels. Chronically high levels of pyroglutamic acid are associated with at least five inborn errors of metabolism including 5-oxoprolinuria, 5-oxoprolinase deficiency, glutathione synthetase deficiency, hawkinsinuria, and propionic acidemia. Pyroglutamic acid is an organic acid. Abnormally high levels of organic acids in the blood (organic acidemia), urine (organic aciduria), the brain, and other tissues lead to general metabolic acidosis. Acidosis typically occurs when arterial pH falls below 7.35. In infants with acidosis, the initial symptoms include poor feeding, vomiting, loss of appetite, weak muscle tone (hypotonia), and lack of energy (lethargy). These can progress to heart, liver, and kidney abnormalities, seizures, coma, and possibly death. These are also the characteristic symptoms of the untreated IEMs mentioned above. Many affected children with organic acidemias experience intellectual disability or delayed development. In adults, acidosis or acidemia is characterized by headaches, confusion, feeling tired, tremors, sleepiness, and seizures. It has been shown that pyroglutamic acid releases GABA from the cerebral cortex and displays anti-anxiety effects in a simple approach-avoidance conflict situation in the rat. In clinical pharmacology experiments, pyroglutamic acid significantly shortens the plasma half-life of ethanol during acute intoxication. | Read more...
|---|
| Structure | InChI=1S/C5H7NO3/c7-4-2-1-3(6-4)5(8)9/h3H,1-2H2,(H,6,7)(H,8,9)/t3-/m0/s1 |
|---|
| Synonyms | | Value | Source |
|---|
| (-)-2-Pyrrolidone-5-carboxylic acid | ChEBI | | (S)-(-)-2-Pyrrolidone-5-carboxylic acid | ChEBI | | (S)-Pyroglutamic acid | ChEBI | | 5-Pyrrolidone-2-carboxylic acid | ChEBI | | L-5-Pyrrolidone-2-carboxylic acid | ChEBI | | L-Pyroglutamic acid | ChEBI | | Pidolic acid | ChEBI | | Pyroglutamate | ChEBI | | 5-oxo-L-Proline | Kegg | | (-)-2-Pyrrolidone-5-carboxylate | Generator | | (S)-(-)-2-Pyrrolidone-5-carboxylate | Generator | | (S)-Pyroglutamate | Generator | | 5-Pyrrolidone-2-carboxylate | Generator | | L-5-Pyrrolidone-2-carboxylate | Generator | | L-Pyroglutamate | Generator | | Pidolate | Generator | | (-)-Pyroglutamate | HMDB | | (-)-Pyroglutamic acid | HMDB | | (5S)-2-Oxopyrrolidine-5-carboxylate | HMDB | | (5S)-2-Oxopyrrolidine-5-carboxylic acid | HMDB | | (S)-(-)-g-Butyrolactam-g-carboxylate | HMDB | | (S)-(-)-g-Butyrolactam-g-carboxylic acid | HMDB | | (S)-(-)-gamma-Butyrolactam-gamma-carboxylate | HMDB | | (S)-(-)-gamma-Butyrolactam-gamma-carboxylic acid | HMDB | | (S)-2-Pyrrolidone-5-carboxylate | HMDB | | (S)-2-Pyrrolidone-5-carboxylic acid | HMDB | | (S)-5-oxo-2-Pyrrolidinecarboxylate | HMDB | | (S)-5-oxo-2-Pyrrolidinecarboxylic acid | HMDB | | 2-L-Pyrrolidone-5-carboxylate | HMDB | | 2-L-Pyrrolidone-5-carboxylic acid | HMDB | | 2-Oxopyrrolidine-5(S)-carboxylate | HMDB | | 2-Oxopyrrolidine-5(S)-carboxylic acid | HMDB | | 2-Pyrrolidinone-5-carboxylate | HMDB | | 2-Pyrrolidinone-5-carboxylic acid | HMDB | | 5-Carboxy-2-pyrrolidinone | HMDB | | 5-L-Oxoproline | HMDB | | 5-Oxoproline | HMDB | | 5-Pyrrolidinone-2-carboxylate | HMDB | | 5-Pyrrolidinone-2-carboxylic acid | HMDB | | Ajidew a 100 | HMDB | | Glutimate | HMDB | | Glutimic acid | HMDB | | Glutiminate | HMDB | | Glutiminic acid | HMDB | | L-2-Pyrrolidone-5-carboxylate | HMDB | | L-2-Pyrrolidone-5-carboxylic acid | HMDB | | L-5-Carboxy-2-pyrrolidinone | HMDB | | L-5-oxo-2-Pyrrolidinecarboxylate | HMDB | | L-5-oxo-2-Pyrrolidinecarboxylic acid | HMDB | | L-5-Oxoproline | HMDB | | L-Glutamic acid g-lactam | HMDB | | L-Glutimate | HMDB | | L-Glutimic acid | HMDB | | L-Glutiminate | HMDB | | L-Glutiminic acid | HMDB | | L-Pyrrolidinonecarboxylate | HMDB | | L-Pyrrolidinonecarboxylic acid | HMDB | | L-Pyrrolidonecarboxylate | HMDB | | L-Pyrrolidonecarboxylic acid | HMDB | | Oxoproline | HMDB | | Oxopyrrolidinecarboxylate | HMDB | | Oxopyrrolidinecarboxylic acid | HMDB | | Pidolidone | HMDB | | Pyrrolidinonecarboxylate | HMDB | | Pyrrolidinonecarboxylic acid | HMDB | | Pyrrolidone-5-carboxylate | HMDB | | Pyrrolidone-5-carboxylic acid | HMDB | | Pyrrolidonecarboxylic acid | HMDB | | 5-Ketoproline | HMDB | | Pidolate, magnesium | HMDB | | 5-Oxopyrrolidine-2-carboxylic acid | HMDB | | Magnesium pidolate | HMDB | | 2-Pyrrolidone-5-carboxylic acid | HMDB | | 5-Oxoprolinate | HMDB | | PCA | HMDB |
| Show more...
|---|
| Chemical Formula | C5H7NO3 |
|---|
| Average Molecular Weight | 129.114 |
|---|
| Monoisotopic Molecular Weight | 129.042593095 |
|---|
| IUPAC Name | (2S)-5-oxopyrrolidine-2-carboxylic acid |
|---|
| Traditional Name | pyroglutamic acid |
|---|
| CAS Registry Number | 98-79-3 |
|---|
| SMILES | OC(=O)[C@@H]1CCC(=O)N1 |
|---|
| InChI Identifier | InChI=1S/C5H7NO3/c7-4-2-1-3(6-4)5(8)9/h3H,1-2H2,(H,6,7)(H,8,9)/t3-/m0/s1 |
|---|
| InChI Key | ODHCTXKNWHHXJC-VKHMYHEASA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as alpha amino acids and derivatives. These are amino acids in which the amino group is attached to the carbon atom immediately adjacent to the carboxylate group (alpha carbon), or a derivative thereof. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Alpha amino acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-amino acid or derivatives
- Pyrroline carboxylic acid
- Pyrroline carboxylic acid or derivatives
- Pyrroline
- Cyclic carboximidic acid
- Lactim
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Propargyl-type 1,3-dipolar organic compound
- Organic 1,3-dipolar compound
- Azacycle
- Organoheterocyclic compound
- Organopnictogen compound
- Organic oxygen compound
- Organooxygen compound
- Organic oxide
- Hydrocarbon derivative
- Carbonyl group
- Organic nitrogen compound
- Organonitrogen compound
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Physiological effect | |
|---|
| Disposition | |
|---|
| Process | |
|---|
| Role | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 476 mg/mL at 13 °C | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Experimental Collision Cross Sections |
|---|
| Predicted Molecular Properties | | Show more...
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Kovats Retention IndicesUnderivatizedDerivatized| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| Pyroglutamic acid,1TMS,isomer #1 | C[Si](C)(C)OC(=O)[C@@H]1CCC(=O)N1 | 1478.9 | Semi standard non polar | 33892256 | | Pyroglutamic acid,1TMS,isomer #2 | C[Si](C)(C)N1C(=O)CC[C@H]1C(=O)O | 1484.5 | Semi standard non polar | 33892256 | | Pyroglutamic acid,2TMS,isomer #1 | C[Si](C)(C)OC(=O)[C@@H]1CCC(=O)N1[Si](C)(C)C | 1494.7 | Semi standard non polar | 33892256 | | Pyroglutamic acid,2TMS,isomer #1 | C[Si](C)(C)OC(=O)[C@@H]1CCC(=O)N1[Si](C)(C)C | 1478.9 | Standard non polar | 33892256 | | Pyroglutamic acid,2TMS,isomer #1 | C[Si](C)(C)OC(=O)[C@@H]1CCC(=O)N1[Si](C)(C)C | 1803.4 | Standard polar | 33892256 | | Pyroglutamic acid,1TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)[C@@H]1CCC(=O)N1 | 1718.7 | Semi standard non polar | 33892256 | | Pyroglutamic acid,1TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)N1C(=O)CC[C@H]1C(=O)O | 1717.9 | Semi standard non polar | 33892256 | | Pyroglutamic acid,2TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)[C@@H]1CCC(=O)N1[Si](C)(C)C(C)(C)C | 1928.0 | Semi standard non polar | 33892256 | | Pyroglutamic acid,2TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)[C@@H]1CCC(=O)N1[Si](C)(C)C(C)(C)C | 1945.3 | Standard non polar | 33892256 | | Pyroglutamic acid,2TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)[C@@H]1CCC(=O)N1[Si](C)(C)C(C)(C)C | 1985.5 | Standard polar | 33892256 |
| Show more...
|---|
| Spectra |
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental GC-MS | GC-MS Spectrum - Pyroglutamic acid GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (2 TMS) | splash10-0ab9-8900000000-f79dc90370ba38f587c9 | 2014-06-16 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Pyroglutamic acid GC-EI-TOF (Non-derivatized) | splash10-0ab9-8900000000-f79dc90370ba38f587c9 | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Pyroglutamic acid GC-EI-TOF (Non-derivatized) | splash10-0a4i-0900000000-90fb43273551aeb9b2c4 | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Pyroglutamic acid GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a6r-9000000000-130a8f31f82e83c4be07 | 2016-09-22 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Pyroglutamic acid GC-MS (1 TMS) - 70eV, Positive | splash10-05fr-9200000000-d69b52257404ab658d5b | 2017-10-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Pyroglutamic acid GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Pyroglutamic acid GC-MS (TMS_1_2) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Pyroglutamic acid GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Pyroglutamic acid GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-001i-9000000000-6c87253da642bb4800df | 2019-05-16 | Not Available | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental LC-MS/MS | LC-MS/MS Spectrum - Pyroglutamic acid Quattro_QQQ 10V, Positive-QTOF (Annotated) | splash10-001i-9500000000-ebc64308ec5d5bdb303e | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Pyroglutamic acid Quattro_QQQ 25V, Positive-QTOF (Annotated) | splash10-053r-9000000000-7377cb17491942e9589c | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Pyroglutamic acid Quattro_QQQ 40V, Positive-QTOF (Annotated) | splash10-053u-9000000000-fcab1396867356ebd6ae | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Pyroglutamic acid LC-ESI-QQ (API3000, Applied Biosystems) 10V, Negative-QTOF | splash10-004i-0900000000-5b0c6536e1b3217b8544 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Pyroglutamic acid LC-ESI-QQ (API3000, Applied Biosystems) 20V, Negative-QTOF | splash10-004i-0900000000-c30ac0bd264c8007ef92 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Pyroglutamic acid LC-ESI-QQ (API3000, Applied Biosystems) 30V, Negative-QTOF | splash10-004i-5900000000-ea3a164653e4235716ae | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Pyroglutamic acid LC-ESI-QQ (API3000, Applied Biosystems) 40V, Negative-QTOF | splash10-0f89-9000000000-f6620738e68f990d0594 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Pyroglutamic acid LC-ESI-QQ (API3000, Applied Biosystems) 50V, Negative-QTOF | splash10-0udi-9000000000-7937bee2e9a6d6b29cbd | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Pyroglutamic acid LC-ESI-QQ (API3000, Applied Biosystems) 10V, Negative-QTOF | splash10-004i-0900000000-f20401903b234914b936 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Pyroglutamic acid LC-ESI-QQ (API3000, Applied Biosystems) 20V, Negative-QTOF | splash10-004i-0900000000-9446bb65e0edd72cfd59 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Pyroglutamic acid LC-ESI-QQ (API3000, Applied Biosystems) 30V, Negative-QTOF | splash10-0059-7900000000-74eccdeb9f0d5fd17614 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Pyroglutamic acid LC-ESI-QQ (API3000, Applied Biosystems) 40V, Negative-QTOF | splash10-0f8a-9000000000-8786a9cd5e488192f34d | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Pyroglutamic acid LC-ESI-QQ (API3000, Applied Biosystems) 50V, Negative-QTOF | splash10-0f6t-9000000000-ebcc1ac4acd525218e80 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Pyroglutamic acid LC-ESI-QQ (API3000, Applied Biosystems) 10V, Positive-QTOF | splash10-01q9-2900000000-754ae9b699ec1b22cd76 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Pyroglutamic acid LC-ESI-QQ (API3000, Applied Biosystems) 20V, Positive-QTOF | splash10-001i-9300000000-eabb8c4dc0d1111e0431 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Pyroglutamic acid LC-ESI-QQ (API3000, Applied Biosystems) 30V, Positive-QTOF | splash10-00lr-9100000000-1dd17702aee7e5bce618 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Pyroglutamic acid LC-ESI-QQ (API3000, Applied Biosystems) 40V, Positive-QTOF | splash10-067i-9000000000-c9669794d3a8746be498 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Pyroglutamic acid LC-ESI-QQ (API3000, Applied Biosystems) 50V, Positive-QTOF | splash10-014i-9000000000-9e103abb0a6ed890051e | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Pyroglutamic acid LC-ESI-QQ (API3000, Applied Biosystems) 10V, Positive-QTOF | splash10-03e9-3900000000-da8cf252285c1d616586 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Pyroglutamic acid LC-ESI-QQ (API3000, Applied Biosystems) 20V, Positive-QTOF | splash10-01q9-9400000000-96a7fe5a81188c49d1ba | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Pyroglutamic acid LC-ESI-QQ (API3000, Applied Biosystems) 30V, Positive-QTOF | splash10-014i-9000000000-356215339a43217dea66 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Pyroglutamic acid LC-ESI-QQ (API3000, Applied Biosystems) 40V, Positive-QTOF | splash10-02vl-9000000000-ed47ec6e675eb338da19 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Pyroglutamic acid LC-ESI-QQ (API3000, Applied Biosystems) 50V, Positive-QTOF | splash10-014i-9000000000-f647da344adbdf7bfb1b | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Pyroglutamic acid LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Positive-QTOF | splash10-001i-9200000000-0bddc68d58c6fb981d1a | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Pyroglutamic acid LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Negative-QTOF | splash10-004i-0900000000-c70c79fa828bbf137ebc | 2012-08-31 | HMDB team, MONA | View Spectrum |
NMR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Experimental 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, experimental) | 2012-12-04 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Experimental 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, H2O, experimental) | 2012-12-05 | Wishart Lab | View Spectrum |
IR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M-H]-) | 2023-02-03 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M+H]+) | 2023-02-03 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M+Na]+) | 2023-02-03 | FELIX lab | View Spectrum |
| Show more...
|---|
| Biological Properties |
|---|
| Cellular Locations | - Cytoplasm (predicted from logP)
|
|---|
| Biospecimen Locations | - Blood
- Cerebrospinal Fluid (CSF)
- Feces
- Saliva
- Sweat
- Urine
|
|---|
| Tissue Locations | - Brain
- Epidermis
- Placenta
- Prostate
|
|---|
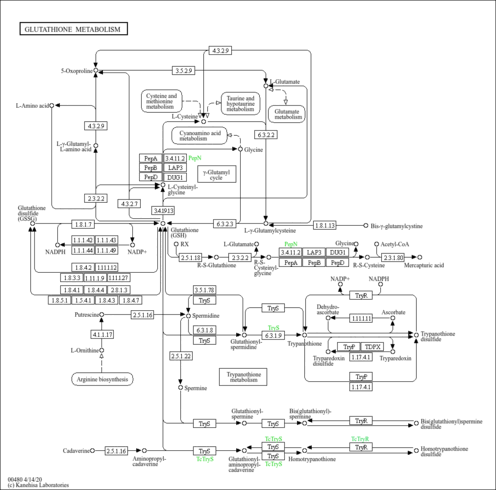
| Pathways | |
|---|
| Normal Concentrations |
|---|
| |
| Blood | Detected and Quantified | 19.5 +/- 3.7 uM | Adult (>18 years old) | Both | Normal | | details | | Blood | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Blood | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 47 +/- 30 uM | Adult (>18 years old) | Both | Normal | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 41.0 +/- 31.0 uM | Adult (>18 years old) | Not Specified | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Infant (0-1 year old) | Both | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Not Specified | Not Specified | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Not Specified | Not Specified | Normal | | details | | Saliva | Detected and Quantified | 5.65 +/- 4.71 uM | Adult (>18 years old) | Female | Normal | | details | | Saliva | Detected and Quantified | 8.70 +/- 7.55 uM | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected and Quantified | 15.0 +/- 32.5 uM | Adult (>18 years old) | Female | Normal | | details | | Saliva | Detected and Quantified | 18.2 +/- 20.9 uM | Adult (>18 years old) | Both | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected and Quantified | 3.82 +/- 5.16 uM | Adult (>18 years old) | Female | Normal | | details | | Saliva | Detected and Quantified | 3.84 +/- 2.81 uM | Adult (>18 years old) | Female | Normal | | details | | Saliva | Detected and Quantified | 0.618 +/- 0.758 uM | Adult (>18 years old) | Both | Normal | | details | | Saliva | Detected and Quantified | 30.19 +/- 31.32 uM | Adult (>18 years old) | Both | Normal | | details | | Sweat | Detected and Quantified | < 10 uM | Adult (60 years old) | Male | Normal | | details | | Sweat | Detected and Quantified | < 10 uM | Adult (40 years old) | Male | Normal | | details | | Urine | Detected and Quantified | 47.641 +/- 19.524 umol/mmol creatinine | Children (1 - 13 years old) | Not Specified | Normal | | details | | Urine | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Urine | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Urine | Detected and Quantified | <10 umol/mmol creatinine | Not Specified | Not Specified | Normal | | details | | Urine | Detected and Quantified | 5-12.3 umol/mmol creatinine | Infant (0-1 year old) | Female | Normal | | details | | Urine | Detected and Quantified | 6.0-22.2 umol/mmol creatinine | Infant (0-1 year old) | Male | Normal | | details | | Urine | Detected and Quantified | 6.7-27.1 umol/mmol creatinine | Infant (0-1 year old) | Female | Normal | | details | | Urine | Detected and Quantified | 8.6-22.0 umol/mmol creatinine | Infant (0-1 year old) | Male | Normal | | details | | Urine | Detected and Quantified | 40.428 +/- 19.331 umol/mmol creatinine | Children (1 - 13 years old) | Not Specified | Normal | | details | | Urine | Detected and Quantified | 21-30 umol/mmol creatinine | Adult (>18 years old) | Female | Normal | | details | | Urine | Detected and Quantified | 20.7 (10.2-32.6) umol/mmol creatinine | Adult (>18 years old) | Both | Normal | | details | | Urine | Detected and Quantified | <79.14 umol/mmol creatinine | Children (1 - 18 years old) | Both | Normal | | details | | Urine | Detected and Quantified | 14.0 +/- 7.2 umol/mmol creatinine | Adult (>18 years old) | Both | Normal | | details | | Urine | Detected and Quantified | 22.99 (19.74-38.23) umol/mmol creatinine | Newborn (0-30 days old) | Both | Normal | | details | | Urine | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Urine | Detected and Quantified | 17-25 umol/mmol creatinine | Adult (>18 years old) | Male | Normal | | details | | Urine | Detected and Quantified | 7.3 (0.1-29.3) umol/mmol creatinine | Newborn (0-30 days old) | Both | Normal | | details | | Urine | Detected and Quantified | 24.1 (3.4-54.2) umol/mmol creatinine | Infant (0-1 year old) | Both | Normal | | details | | Urine | Detected and Quantified | 5.3 (2.9-10.4) umol/mmol creatinine | Children (1-13 years old) | Both | Normal | | details | | Urine | Detected and Quantified | 3.1 (1.9-11.3) umol/mmol creatinine | Adolescent (13-18 years old) | Both | Normal | | details | | Urine | Detected and Quantified | <7.6 umol/mmol creatinine | Children (1 - 13 years old) | Both | Normal | | details | | Urine | Detected and Quantified | 18.6 (4.5-24.9) umol/mmol creatinine | Adult (>18 years old) | Both | Normal | | details | | Urine | Detected and Quantified | 5-44 umol/mmol creatinine | Newborn (0-30 days old) | Both | Normal | | details |
| Show more...
|---|
| Abnormal Concentrations |
|---|
| |
| Blood | Detected and Quantified | 87.00 (13.00-161.00) uM | Adult (>18 years old) | Both | Glutathione synthetase deficiency | | details | | Blood | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Schizophrenia | | details | | Blood | Detected and Quantified | 3.5 (3.00-4.00) uM | Children (1-13 years old) | Both | Glutathione synthetase deficiency | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 2326.0 uM | Adult (>18 years old) | Both | Pyroglutamic aciduria | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Colorectal cancer | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Colorectal Cancer | | details | | Feces | Detected but not Quantified | Not Quantified | Not Specified | Not Specified | Cryptosporidium infection | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Irritable bowel syndrome | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Colorectal cancer | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Ulcerative colitis | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Supragingival Calculus | | details | | Saliva | Detected and Quantified | 0.481 +/- 0.853 uM | Adult (>18 years old) | Both | Dental caries | | details | | Urine | Detected and Quantified | 28.8 (3.4-54.2) umol/mmol creatinine | Adult (>18 years old) | Both | 5-oxoprolinase deficiency | | details | | Urine | Detected and Quantified | 3500.0 (1000.0-6000.0) umol/mmol creatinine | Children (1-13 years old) | Both | 5-Oxoprolinase Deficiency | | details | | Urine | Detected and Quantified | 7931-13208 umol/mmol creatinine | Children (1-13 years old) | Male | Pyroglutamic aciduria | | details | | Urine | Detected and Quantified | 9-7282 umol/mmol creatinine | Infant (0-1 year old) | Female | Pyroglutamic aciduria | | details | | Urine | Detected and Quantified | 34.75 +/- 17.53 umol/mmol creatinine | Children (1 - 13 years old) | Not Specified | Eosinophilic esophagitis | | details | | Urine | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Autosomal dominant polycystic kidney disease (ADPKD) | | details | | Urine | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Bladder cancer | | details | | Urine | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Schizophrenia | | details | | Urine | Detected and Quantified | 1.9 umol/mmol creatinine | Adult (>18 years old) | Both | Pyroglutamic aciduria | | details | | Urine | Detected and Quantified | 5570 umol/mmol creatinine | Children (1-13 years old) | Not Specified | 5-Oxoprolinase Deficiency | | details | | Urine | Detected and Quantified | 1980 - 6040 umol/mmol creatinine | Infant (0-1 year old) | Not Specified | 5-Oxoprolinase Deficiency | | details | | Urine | Detected and Quantified | 137.6 - 4092.79 umol/mmol creatinine | Children (1 - 13 years old) | Both | Glutathione synthetase deficiency | | details | | Urine | Detected and Quantified | 47.522 +/- 35.017 umol/mmol creatinine | Children (1 - 13 years old) | Not Specified | Eosinophilic esophagitis | | details | | Urine | Detected and Quantified | 80.794 +/- 83.738 umol/mmol creatinine | Children (1 - 13 years old) | Not Specified | Gastroesophageal reflux disease | | details |
| Show more...
|---|
| Associated Disorders and Diseases |
|---|
| Disease References | | Glutathione synthetase deficiency |
|---|
- Li X, Ding Y, Liu Y, Ma Y, Song J, Wang Q, Yang Y: Five Chinese patients with 5-oxoprolinuria due to glutathione synthetase and 5-oxoprolinase deficiencies. Brain Dev. 2015 Nov;37(10):952-9. doi: 10.1016/j.braindev.2015.03.005. Epub 2015 Apr 4. [PubMed:25851806 ]
- G.Frauendienst-Egger, Friedrich K. Trefz (2017). MetaGene: Metabolic & Genetic Information Center (MIC: http://www.metagene.de). METAGENE consortium.
| | Schizophrenia |
|---|
- Yang J, Chen T, Sun L, Zhao Z, Qi X, Zhou K, Cao Y, Wang X, Qiu Y, Su M, Zhao A, Wang P, Yang P, Wu J, Feng G, He L, Jia W, Wan C: Potential metabolite markers of schizophrenia. Mol Psychiatry. 2013 Jan;18(1):67-78. doi: 10.1038/mp.2011.131. Epub 2011 Oct 25. [PubMed:22024767 ]
| | Irritable bowel syndrome |
|---|
- Ponnusamy K, Choi JN, Kim J, Lee SY, Lee CH: Microbial community and metabolomic comparison of irritable bowel syndrome faeces. J Med Microbiol. 2011 Jun;60(Pt 6):817-27. doi: 10.1099/jmm.0.028126-0. Epub 2011 Feb 17. [PubMed:21330412 ]
| | Colorectal cancer |
|---|
- Brown DG, Rao S, Weir TL, O'Malia J, Bazan M, Brown RJ, Ryan EP: Metabolomics and metabolic pathway networks from human colorectal cancers, adjacent mucosa, and stool. Cancer Metab. 2016 Jun 6;4:11. doi: 10.1186/s40170-016-0151-y. eCollection 2016. [PubMed:27275383 ]
- Sinha R, Ahn J, Sampson JN, Shi J, Yu G, Xiong X, Hayes RB, Goedert JJ: Fecal Microbiota, Fecal Metabolome, and Colorectal Cancer Interrelations. PLoS One. 2016 Mar 25;11(3):e0152126. doi: 10.1371/journal.pone.0152126. eCollection 2016. [PubMed:27015276 ]
- Goedert JJ, Sampson JN, Moore SC, Xiao Q, Xiong X, Hayes RB, Ahn J, Shi J, Sinha R: Fecal metabolomics: assay performance and association with colorectal cancer. Carcinogenesis. 2014 Sep;35(9):2089-96. doi: 10.1093/carcin/bgu131. Epub 2014 Jul 18. [PubMed:25037050 ]
| | Ulcerative colitis |
|---|
- Azario I, Pievani A, Del Priore F, Antolini L, Santi L, Corsi A, Cardinale L, Sawamoto K, Kubaski F, Gentner B, Bernardo ME, Valsecchi MG, Riminucci M, Tomatsu S, Aiuti A, Biondi A, Serafini M: Neonatal umbilical cord blood transplantation halts skeletal disease progression in the murine model of MPS-I. Sci Rep. 2017 Aug 25;7(1):9473. doi: 10.1038/s41598-017-09958-9. [PubMed:28842642 ]
| | Supragingival Calculus |
|---|
- Liebsch C, Pitchika V, Pink C, Samietz S, Kastenmuller G, Artati A, Suhre K, Adamski J, Nauck M, Volzke H, Friedrich N, Kocher T, Holtfreter B, Pietzner M: The Saliva Metabolome in Association to Oral Health Status. J Dent Res. 2019 Jun;98(6):642-651. doi: 10.1177/0022034519842853. Epub 2019 Apr 26. [PubMed:31026179 ]
| | 5-oxoprolinase deficiency |
|---|
- Calpena E, Deshpande AA, Yap S, Kumar A, Manning NJ, Bachhawat AK, Espinos C: New insights into the genetics of 5-oxoprolinase deficiency and further evidence that it is a benign biochemical condition. Eur J Pediatr. 2015 Mar;174(3):407-11. doi: 10.1007/s00431-014-2397-0. Epub 2014 Aug 17. [PubMed:25129617 ]
- Mayatepek E: 5-Oxoprolinuria in patients with and without defects in the gamma-glutamyl cycle. Eur J Pediatr. 1999 Mar;158(3):221-5. [PubMed:10094443 ]
- G.Frauendienst-Egger, Friedrich K. Trefz (2017). MetaGene: Metabolic & Genetic Information Center (MIC: http://www.metagene.de). METAGENE consortium.
| | Autosomal dominant polycystic kidney disease |
|---|
- Gronwald W, Klein MS, Zeltner R, Schulze BD, Reinhold SW, Deutschmann M, Immervoll AK, Boger CA, Banas B, Eckardt KU, Oefner PJ: Detection of autosomal dominant polycystic kidney disease by NMR spectroscopic fingerprinting of urine. Kidney Int. 2011 Jun;79(11):1244-53. doi: 10.1038/ki.2011.30. Epub 2011 Mar 9. [PubMed:21389975 ]
| | Eosinophilic esophagitis |
|---|
- Slae, M., Huynh, H., Wishart, D.S. (2014). Analysis of 30 normal pediatric urine samples via NMR spectroscopy (unpublished work). NA.
|
|
|---|
| Associated OMIM IDs | - 266130 (Glutathione synthetase deficiency)
- 181500 (Schizophrenia)
- 114500 (Colorectal cancer)
- 260005 (5-oxoprolinase deficiency)
- 601313 (Autosomal dominant polycystic kidney disease)
- 610247 (Eosinophilic esophagitis)
|
|---|
| External Links |
|---|
| DrugBank ID | DB03088 |
|---|
| Phenol Explorer Compound ID | Not Available |
|---|
| FooDB ID | FDB014506 |
|---|
| KNApSAcK ID | C00007403 |
|---|
| Chemspider ID | Not Available |
|---|
| KEGG Compound ID | C01879 |
|---|
| BioCyc ID | 5-OXOPROLINE |
|---|
| BiGG ID | Not Available |
|---|
| Wikipedia Link | Pyroglutamic_acid |
|---|
| METLIN ID | Not Available |
|---|
| PubChem Compound | 7405 |
|---|
| PDB ID | Not Available |
|---|
| ChEBI ID | 18183 |
|---|
| Food Biomarker Ontology | Not Available |
|---|
| VMH ID | 5OXPRO |
|---|
| MarkerDB ID | MDB00000129 |
|---|
| Good Scents ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Pumpor, Ksenia; Boettcher, Christoph; Fehn, Susanna; Burger, Klaus. Hexafluoroacetone as protecting and activating reagent: an efficient strategy for activation of pyroglutamic acid and homologs.Heterocycles (2003), 61 259-269. |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| General References | - Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, Laxman B, Mehra R, Lonigro RJ, Li Y, Nyati MK, Ahsan A, Kalyana-Sundaram S, Han B, Cao X, Byun J, Omenn GS, Ghosh D, Pennathur S, Alexander DC, Berger A, Shuster JR, Wei JT, Varambally S, Beecher C, Chinnaiyan AM: Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009 Feb 12;457(7231):910-4. doi: 10.1038/nature07762. [PubMed:19212411 ]
- Guneral F, Bachmann C: Age-related reference values for urinary organic acids in a healthy Turkish pediatric population. Clin Chem. 1994 Jun;40(6):862-6. [PubMed:8087979 ]
- Hoffmann GF, Meier-Augenstein W, Stockler S, Surtees R, Rating D, Nyhan WL: Physiology and pathophysiology of organic acids in cerebrospinal fluid. J Inherit Metab Dis. 1993;16(4):648-69. [PubMed:8412012 ]
- Jellum E, Stokke O, Eldjarn L: Combined use of gas chromatography, mass spectrometry, and computer in diagnosis and studies of metabolic disorders. Clin Chem. 1972 Aug;18(8):800-9. [PubMed:4557757 ]
- Wevers RA, Engelke U, Heerschap A: High-resolution 1H-NMR spectroscopy of blood plasma for metabolic studies. Clin Chem. 1994 Jul;40(7 Pt 1):1245-50. [PubMed:8013094 ]
- Manning NJ, Davies NP, Olpin SE, Carpenter KH, Smith MF, Pollitt RJ, Duncan SL, Larsson A, Carlsson B: Prenatal diagnosis of glutathione synthase deficiency. Prenat Diagn. 1994 Jun;14(6):475-8. [PubMed:7937585 ]
- Caspers PJ, Lucassen GW, Carter EA, Bruining HA, Puppels GJ: In vivo confocal Raman microspectroscopy of the skin: noninvasive determination of molecular concentration profiles. J Invest Dermatol. 2001 Mar;116(3):434-42. [PubMed:11231318 ]
- Hussain Z, Lannigan R, Stoakes L: A new approach for presumptive identification of clinically important streptococci. Zentralbl Bakteriol Mikrobiol Hyg A. 1984 Oct;258(1):74-9. [PubMed:6441390 ]
- Creer MH, Lau BW, Jones JD, Chan KM: Pyroglutamic acidemia in an adult patient. Clin Chem. 1989 Apr;35(4):684-6. [PubMed:2702756 ]
- Hammond JW, Potter M, Truscott R, Wilcken B: gamma-Glutamylglutamine identified in plasma and cerebrospinal fluid from hyperammonaemic patients. Clin Chim Acta. 1990 Dec 24;194(2-3):173-83. [PubMed:2093471 ]
- Uhlhaas S, Lange H: Striatal deficiency of L-pyroglutamic acid in Huntington's disease is accompanied by increased plasma levels. Brain Res. 1988 Aug 2;457(1):196-9. [PubMed:2971422 ]
- Croal BL, Glen AC, Kelly CJ, Logan RW: Transient 5-oxoprolinuria (pyroglutamic aciduria) with systemic acidosis in an adult receiving antibiotic therapy. Clin Chem. 1998 Feb;44(2):336-40. [PubMed:9474033 ]
- Winslow JW, Shih A, Bourell JH, Weiss G, Reed B, Stults JT, Goldsmith LT: Human seminal relaxin is a product of the same gene as human luteal relaxin. Endocrinology. 1992 May;130(5):2660-8. [PubMed:1572287 ]
- Erasmus E, Mienie LJ, de Vries WN, de Wet WJ, Carlsson B, Larsson A: Prenatal analysis in two suspected cases of glutathione synthetase deficiency. J Inherit Metab Dis. 1993;16(5):837-43. [PubMed:8295398 ]
- Palekar AG, Tate SS, Sullivan JF, Meister A: Accumulation of 50oxo-L-proline and 5-oxo-D-proline in the blood plasma in end stage renal disease. Biochem Med. 1975 Nov;14(3):339-45. [PubMed:1225334 ]
- Monsigny M, Rondanino C, Duverger E, Fajac I, Roche AC: Glyco-dependent nuclear import of glycoproteins, glycoplexes and glycosylated plasmids. Biochim Biophys Acta. 2004 Jul 6;1673(1-2):94-103. [PubMed:15238252 ]
- Thiele I, Swainston N, Fleming RM, Hoppe A, Sahoo S, Aurich MK, Haraldsdottir H, Mo ML, Rolfsson O, Stobbe MD, Thorleifsson SG, Agren R, Bolling C, Bordel S, Chavali AK, Dobson P, Dunn WB, Endler L, Hala D, Hucka M, Hull D, Jameson D, Jamshidi N, Jonsson JJ, Juty N, Keating S, Nookaew I, Le Novere N, Malys N, Mazein A, Papin JA, Price ND, Selkov E Sr, Sigurdsson MI, Simeonidis E, Sonnenschein N, Smallbone K, Sorokin A, van Beek JH, Weichart D, Goryanin I, Nielsen J, Westerhoff HV, Kell DB, Mendes P, Palsson BO: A community-driven global reconstruction of human metabolism. Nat Biotechnol. 2013 May;31(5):419-25. doi: 10.1038/nbt.2488. Epub 2013 Mar 3. [PubMed:23455439 ]
- Elshenawy S, Pinney SE, Stuart T, Doulias PT, Zura G, Parry S, Elovitz MA, Bennett MJ, Bansal A, Strauss JF 3rd, Ischiropoulos H, Simmons RA: The Metabolomic Signature of the Placenta in Spontaneous Preterm Birth. Int J Mol Sci. 2020 Feb 4;21(3). pii: ijms21031043. doi: 10.3390/ijms21031043. [PubMed:32033212 ]
| Show more...
|---|