| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Expected but not Quantified |
|---|
| Creation Date | 2008-08-12 13:32:54 UTC |
|---|
| Update Date | 2022-03-07 02:49:33 UTC |
|---|
| HMDB ID | HMDB0006807 |
|---|
| Secondary Accession Numbers | - HMDB0002125
- HMDB02125
- HMDB06807
|
|---|
| Metabolite Identification |
|---|
| Common Name | 3-Hydroxypropionyl-CoA |
|---|
| Description | 3-Hydroxypropionyl-CoA, also known as beta-hydroxypropionyl-CoA, belongs to the class of organic compounds known as acyl-CoAs. These are organic compounds containing a coenzyme A substructure linked to an acyl chain. Thus, 3-hydroxypropionyl-CoA is considered to be a fatty ester lipid molecule. 3-Hydroxypropionyl-CoA is a very hydrophobic molecule, practically insoluble (in water), and relatively neutral. 3-Hydroxypropionyl-CoA is an intermediate in beta-Alanine metabolism. It can be produced from 3-hydroxypropanoic acid via the enzyme 3-hydroxyisobutyryl-coenzyme A hydrolase (EC 3.1.2.4) or it can be generated from acrylyl-CoA via the enzyme enoyl-CoA hydratase (EC 4.2.1.17). Acrylyl-CoA is derived from propionyl-CoA. |
|---|
| Structure | CC(C)(COP(O)(=O)OP(O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1OP(O)(O)=O)N1C=NC2=C1N=CN=C2N)[C@@H](O)C(=O)NCCC(=O)NCCSC(=O)CCO InChI=1S/C24H40N7O18P3S/c1-24(2,19(36)22(37)27-5-3-14(33)26-6-8-53-15(34)4-7-32)10-46-52(43,44)49-51(41,42)45-9-13-18(48-50(38,39)40)17(35)23(47-13)31-12-30-16-20(25)28-11-29-21(16)31/h11-13,17-19,23,32,35-36H,3-10H2,1-2H3,(H,26,33)(H,27,37)(H,41,42)(H,43,44)(H2,25,28,29)(H2,38,39,40)/t13-,17-,18-,19+,23-/m1/s1 |
|---|
| Synonyms | | Value | Source |
|---|
| 3-Hydroxypropanoyl coenzymea | ChEBI | | 3-Hydroxypropionyl coenzyme A | ChEBI | | beta-Hydroxypropionyl-CoA | ChEBI | | 3-Hydroxypropanoyl-CoA | Kegg | | b-Hydroxypropionyl-CoA | Generator | | Β-hydroxypropionyl-CoA | Generator | | 3-Hydroxypropanoyl coenzyme A | HMDB | | 3-Hydroxypropanoyl-coenzyme A | HMDB | | 3-Hydroxypropionyl-coenzyme A | HMDB | | beta-Hydroxypropionyl-coenzyme A | HMDB | | 3-Hydroxypropionyl-CoA | ChEBI |
|
|---|
| Chemical Formula | C24H40N7O18P3S |
|---|
| Average Molecular Weight | 839.6 |
|---|
| Monoisotopic Molecular Weight | 839.136339644 |
|---|
| IUPAC Name | {[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-hydroxy-2-({[hydroxy({hydroxy[(3R)-3-hydroxy-3-{[2-({2-[(3-hydroxypropanoyl)sulfanyl]ethyl}carbamoyl)ethyl]carbamoyl}-2,2-dimethylpropoxy]phosphoryl}oxy)phosphoryl]oxy}methyl)oxolan-3-yl]oxy}phosphonic acid |
|---|
| Traditional Name | 3-hydroxypropionyl coenzyme A |
|---|
| CAS Registry Number | 157786-88-4 |
|---|
| SMILES | CC(C)(COP(O)(=O)OP(O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1OP(O)(O)=O)N1C=NC2=C1N=CN=C2N)[C@@H](O)C(=O)NCCC(=O)NCCSC(=O)CCO |
|---|
| InChI Identifier | InChI=1S/C24H40N7O18P3S/c1-24(2,19(36)22(37)27-5-3-14(33)26-6-8-53-15(34)4-7-32)10-46-52(43,44)49-51(41,42)45-9-13-18(48-50(38,39)40)17(35)23(47-13)31-12-30-16-20(25)28-11-29-21(16)31/h11-13,17-19,23,32,35-36H,3-10H2,1-2H3,(H,26,33)(H,27,37)(H,41,42)(H,43,44)(H2,25,28,29)(H2,38,39,40)/t13-,17-,18-,19+,23-/m1/s1 |
|---|
| InChI Key | BERBFZCUSMQABM-IEXPHMLFSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as acyl coas. These are organic compounds containing a coenzyme A substructure linked to an acyl chain. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty acyl thioesters |
|---|
| Direct Parent | Acyl CoAs |
|---|
| Alternative Parents | |
|---|
| Substituents | - Coenzyme a or derivatives
- Purine ribonucleoside diphosphate
- Purine ribonucleoside bisphosphate
- Purine ribonucleoside 3',5'-bisphosphate
- Ribonucleoside 3'-phosphate
- Pentose-5-phosphate
- Pentose phosphate
- Beta amino acid or derivatives
- Glycosyl compound
- N-glycosyl compound
- 6-aminopurine
- Monosaccharide phosphate
- Organic pyrophosphate
- Imidazopyrimidine
- Purine
- Monoalkyl phosphate
- Aminopyrimidine
- Fatty amide
- Monosaccharide
- N-acyl-amine
- N-substituted imidazole
- Organic phosphoric acid derivative
- Imidolactam
- Phosphoric acid ester
- Alkyl phosphate
- Pyrimidine
- Tetrahydrofuran
- Azole
- Heteroaromatic compound
- Imidazole
- Carbothioic s-ester
- Secondary carboxylic acid amide
- Secondary alcohol
- Thiocarboxylic acid ester
- Carboxamide group
- Amino acid or derivatives
- Thiocarboxylic acid or derivatives
- Sulfenyl compound
- Carboxylic acid derivative
- Organoheterocyclic compound
- Oxacycle
- Azacycle
- Hydrocarbon derivative
- Organic nitrogen compound
- Organic oxide
- Organosulfur compound
- Organonitrogen compound
- Primary amine
- Organopnictogen compound
- Carbonyl group
- Organic oxygen compound
- Primary alcohol
- Organooxygen compound
- Alcohol
- Amine
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Physiological effect | Not Available |
|---|
| Disposition | |
|---|
| Process | |
|---|
| Role | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | 121.5 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Not Available |
|---|
| Predicted Molecular Properties | | Show more...
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Kovats Retention IndicesUnderivatized | Show more...
|---|
| Spectra |
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| MS | Mass Spectrum (Electron Ionization) | Not Available | 2022-08-06 | Not Available | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3-Hydroxypropionyl-CoA 10V, Positive-QTOF | Not Available | 2020-06-30 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3-Hydroxypropionyl-CoA 20V, Positive-QTOF | Not Available | 2020-06-30 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3-Hydroxypropionyl-CoA 40V, Positive-QTOF | Not Available | 2020-06-30 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3-Hydroxypropionyl-CoA 10V, Negative-QTOF | Not Available | 2020-06-30 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3-Hydroxypropionyl-CoA 20V, Negative-QTOF | Not Available | 2020-06-30 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3-Hydroxypropionyl-CoA 40V, Negative-QTOF | Not Available | 2020-06-30 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3-Hydroxypropionyl-CoA 10V, Positive-QTOF | splash10-0006-0300000090-a82d2d516ca943d72c80 | 2021-09-23 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3-Hydroxypropionyl-CoA 20V, Positive-QTOF | splash10-000i-1902000020-b39da26a998aa34c50d8 | 2021-09-23 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3-Hydroxypropionyl-CoA 40V, Positive-QTOF | splash10-001i-1209000000-fa0e10752c42179b290b | 2021-09-23 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3-Hydroxypropionyl-CoA 10V, Negative-QTOF | splash10-000i-0000000190-d665b090f2618519db91 | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3-Hydroxypropionyl-CoA 20V, Negative-QTOF | splash10-000w-9100002850-e526e5730e2eaa7d5372 | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3-Hydroxypropionyl-CoA 40V, Negative-QTOF | splash10-002f-9002304800-0d1446692ec238dd61c4 | 2021-09-25 | Wishart Lab | View Spectrum |
NMR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum |
| Show more...
|---|
| Biological Properties |
|---|
| Cellular Locations | |
|---|
| Biospecimen Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
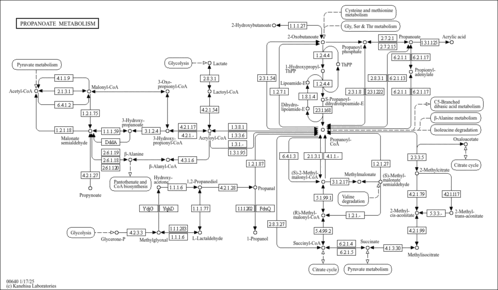
| Pathways | |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| Associated Disorders and Diseases |
|---|
| Disease References | None |
|---|
| Associated OMIM IDs | None |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| Phenol Explorer Compound ID | Not Available |
|---|
| FooDB ID | FDB024093 |
|---|
| KNApSAcK ID | Not Available |
|---|
| Chemspider ID | 10140164 |
|---|
| KEGG Compound ID | C05668 |
|---|
| BioCyc ID | 3-HYDROXY-PROPIONYL-COA |
|---|
| BiGG ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PubChem Compound | 11966171 |
|---|
| PDB ID | Not Available |
|---|
| ChEBI ID | 27762 |
|---|
| Food Biomarker Ontology | Not Available |
|---|
| VMH ID | 3HPCOA |
|---|
| MarkerDB ID | Not Available |
|---|
| Good Scents ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| General References | - Brass EP, Tahiliani AG, Allen RH, Stabler SP: Coenzyme A metabolism in vitamin B-12-deficient rats. J Nutr. 1990 Mar;120(3):290-7. [PubMed:2319347 ]
- DeBuysere MS, Olson MS: The analysis of acyl-coenzyme A derivatives by reverse-phase high-performance liquid chromatography. Anal Biochem. 1983 Sep;133(2):373-9. [PubMed:6638498 ]
- Bucholtz ML, Light RJ: Acetylation of 13-sophorosyloxydocosanoic acid by an acetyltransferase purified from Candida bogoriensis. J Biol Chem. 1976 Jan 25;251(2):424-30. [PubMed:1245481 ]
|
|---|