| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Expected but not Quantified |
|---|
| Creation Date | 2009-07-13 14:03:09 UTC |
|---|
| Update Date | 2022-03-07 02:51:26 UTC |
|---|
| HMDB ID | HMDB0012459 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 7 alpha,26-Dihydroxy-4-cholesten-3-one |
|---|
| Description | 7 alpha,26-Dihydroxy-4-cholesten-3-one belongs to the class of organic compounds known as dihydroxy bile acids, alcohols and derivatives. Dihydroxy bile acids, alcohols and derivatives are compounds containing or derived from a bile acid or alcohol, and which bears exactly two carboxylic acid groups. Based on a literature review a small amount of articles have been published on 7 alpha,26-Dihydroxy-4-cholesten-3-one. |
|---|
| Structure | CC(CO)CCC[C@@H](C)C1CCC2C3[C@H](O)CC4=CC(=O)CC[C@]4(C)C3CC[C@]12C InChI=1S/C27H44O3/c1-17(16-28)6-5-7-18(2)21-8-9-22-25-23(11-13-27(21,22)4)26(3)12-10-20(29)14-19(26)15-24(25)30/h14,17-18,21-25,28,30H,5-13,15-16H2,1-4H3/t17?,18-,21?,22?,23?,24-,25?,26+,27-/m1/s1 |
|---|
| Synonyms | | Value | Source |
|---|
| 7 a,26-Dihydroxy-4-cholesten-3-one | Generator | | 7 Α,26-dihydroxy-4-cholesten-3-one | Generator | | (7alpha)-7,26-Dihydroxy-cholest-4-en-3-one | HMDB | | 4-Cholesten-7alpha,26-diol-3-one | HMDB | | 7,26-Dhxyclso | HMDB, MeSH | | 7alpha,26-Dihydroxy-4-cholesten-3-one | HMDB | | 7alpha,26-Dihydroxycholest-4-en-3-one | HMDB |
|
|---|
| Chemical Formula | C27H44O3 |
|---|
| Average Molecular Weight | 416.6365 |
|---|
| Monoisotopic Molecular Weight | 416.329045274 |
|---|
| IUPAC Name | (2R,9R,15R)-9-hydroxy-14-[(2R)-7-hydroxy-6-methylheptan-2-yl]-2,15-dimethyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadec-6-en-5-one |
|---|
| Traditional Name | (2R,9R,15R)-9-hydroxy-14-[(2R)-7-hydroxy-6-methylheptan-2-yl]-2,15-dimethyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadec-6-en-5-one |
|---|
| CAS Registry Number | 4675-38-1 |
|---|
| SMILES | CC(CO)CCC[C@@H](C)C1CCC2C3[C@H](O)CC4=CC(=O)CC[C@]4(C)C3CC[C@]12C |
|---|
| InChI Identifier | InChI=1S/C27H44O3/c1-17(16-28)6-5-7-18(2)21-8-9-22-25-23(11-13-27(21,22)4)26(3)12-10-20(29)14-19(26)15-24(25)30/h14,17-18,21-25,28,30H,5-13,15-16H2,1-4H3/t17?,18-,21?,22?,23?,24-,25?,26+,27-/m1/s1 |
|---|
| InChI Key | KVJVJJWIEXCECB-JZGXDVPNSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as dihydroxy bile acids, alcohols and derivatives. Dihydroxy bile acids, alcohols and derivatives are compounds containing or derived from a bile acid or alcohol, and which bears exactly two carboxylic acid groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Bile acids, alcohols and derivatives |
|---|
| Direct Parent | Dihydroxy bile acids, alcohols and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - 26-hydroxysteroid
- Dihydroxy bile acid, alcohol, or derivatives
- 3-oxo-delta-4-steroid
- 3-oxosteroid
- 7-hydroxysteroid
- Oxosteroid
- Hydroxysteroid
- Delta-4-steroid
- Fatty alcohol
- Cyclohexenone
- Fatty acyl
- Cyclic alcohol
- Secondary alcohol
- Cyclic ketone
- Ketone
- Organooxygen compound
- Primary alcohol
- Hydrocarbon derivative
- Organic oxygen compound
- Carbonyl group
- Alcohol
- Organic oxide
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Not Available | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Not Available |
|---|
| Predicted Molecular Properties | | Show more...
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Kovats Retention IndicesUnderivatizedDerivatized| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| 7 alpha,26-Dihydroxy-4-cholesten-3-one,1TMS,isomer #1 | CC(CCC[C@@H](C)C1CCC2C3C(CC[C@@]21C)[C@@]1(C)CCC(=O)C=C1C[C@H]3O)CO[Si](C)(C)C | 3646.0 | Semi standard non polar | 33892256 | | 7 alpha,26-Dihydroxy-4-cholesten-3-one,1TMS,isomer #2 | CC(CO)CCC[C@@H](C)C1CCC2C3C(CC[C@@]21C)[C@@]1(C)CCC(=O)C=C1C[C@H]3O[Si](C)(C)C | 3642.8 | Semi standard non polar | 33892256 | | 7 alpha,26-Dihydroxy-4-cholesten-3-one,1TMS,isomer #3 | CC(CO)CCC[C@@H](C)C1CCC2C3C(CC[C@@]21C)[C@@]1(C)CC=C(O[Si](C)(C)C)C=C1C[C@H]3O | 3540.0 | Semi standard non polar | 33892256 | | 7 alpha,26-Dihydroxy-4-cholesten-3-one,2TMS,isomer #1 | CC(CCC[C@@H](C)C1CCC2C3C(CC[C@@]21C)[C@@]1(C)CCC(=O)C=C1C[C@H]3O[Si](C)(C)C)CO[Si](C)(C)C | 3647.6 | Semi standard non polar | 33892256 | | 7 alpha,26-Dihydroxy-4-cholesten-3-one,2TMS,isomer #2 | CC(CCC[C@@H](C)C1CCC2C3C(CC[C@@]21C)[C@@]1(C)CC=C(O[Si](C)(C)C)C=C1C[C@H]3O)CO[Si](C)(C)C | 3549.5 | Semi standard non polar | 33892256 | | 7 alpha,26-Dihydroxy-4-cholesten-3-one,2TMS,isomer #3 | CC(CO)CCC[C@@H](C)C1CCC2C3C(CC[C@@]21C)[C@@]1(C)CC=C(O[Si](C)(C)C)C=C1C[C@H]3O[Si](C)(C)C | 3507.0 | Semi standard non polar | 33892256 | | 7 alpha,26-Dihydroxy-4-cholesten-3-one,3TMS,isomer #1 | CC(CCC[C@@H](C)C1CCC2C3C(CC[C@@]21C)[C@@]1(C)CC=C(O[Si](C)(C)C)C=C1C[C@H]3O[Si](C)(C)C)CO[Si](C)(C)C | 3464.5 | Semi standard non polar | 33892256 | | 7 alpha,26-Dihydroxy-4-cholesten-3-one,3TMS,isomer #1 | CC(CCC[C@@H](C)C1CCC2C3C(CC[C@@]21C)[C@@]1(C)CC=C(O[Si](C)(C)C)C=C1C[C@H]3O[Si](C)(C)C)CO[Si](C)(C)C | 3601.7 | Standard non polar | 33892256 | | 7 alpha,26-Dihydroxy-4-cholesten-3-one,3TMS,isomer #1 | CC(CCC[C@@H](C)C1CCC2C3C(CC[C@@]21C)[C@@]1(C)CC=C(O[Si](C)(C)C)C=C1C[C@H]3O[Si](C)(C)C)CO[Si](C)(C)C | 3682.2 | Standard polar | 33892256 | | 7 alpha,26-Dihydroxy-4-cholesten-3-one,1TBDMS,isomer #1 | CC(CCC[C@@H](C)C1CCC2C3C(CC[C@@]21C)[C@@]1(C)CCC(=O)C=C1C[C@H]3O)CO[Si](C)(C)C(C)(C)C | 3889.9 | Semi standard non polar | 33892256 | | 7 alpha,26-Dihydroxy-4-cholesten-3-one,1TBDMS,isomer #2 | CC(CO)CCC[C@@H](C)C1CCC2C3C(CC[C@@]21C)[C@@]1(C)CCC(=O)C=C1C[C@H]3O[Si](C)(C)C(C)(C)C | 3851.5 | Semi standard non polar | 33892256 | | 7 alpha,26-Dihydroxy-4-cholesten-3-one,1TBDMS,isomer #3 | CC(CO)CCC[C@@H](C)C1CCC2C3C(CC[C@@]21C)[C@@]1(C)CC=C(O[Si](C)(C)C(C)(C)C)C=C1C[C@H]3O | 3767.9 | Semi standard non polar | 33892256 | | 7 alpha,26-Dihydroxy-4-cholesten-3-one,2TBDMS,isomer #1 | CC(CCC[C@@H](C)C1CCC2C3C(CC[C@@]21C)[C@@]1(C)CCC(=O)C=C1C[C@H]3O[Si](C)(C)C(C)(C)C)CO[Si](C)(C)C(C)(C)C | 4089.2 | Semi standard non polar | 33892256 | | 7 alpha,26-Dihydroxy-4-cholesten-3-one,2TBDMS,isomer #2 | CC(CCC[C@@H](C)C1CCC2C3C(CC[C@@]21C)[C@@]1(C)CC=C(O[Si](C)(C)C(C)(C)C)C=C1C[C@H]3O)CO[Si](C)(C)C(C)(C)C | 4014.1 | Semi standard non polar | 33892256 | | 7 alpha,26-Dihydroxy-4-cholesten-3-one,2TBDMS,isomer #3 | CC(CO)CCC[C@@H](C)C1CCC2C3C(CC[C@@]21C)[C@@]1(C)CC=C(O[Si](C)(C)C(C)(C)C)C=C1C[C@H]3O[Si](C)(C)C(C)(C)C | 3925.2 | Semi standard non polar | 33892256 | | 7 alpha,26-Dihydroxy-4-cholesten-3-one,3TBDMS,isomer #1 | CC(CCC[C@@H](C)C1CCC2C3C(CC[C@@]21C)[C@@]1(C)CC=C(O[Si](C)(C)C(C)(C)C)C=C1C[C@H]3O[Si](C)(C)C(C)(C)C)CO[Si](C)(C)C(C)(C)C | 4126.6 | Semi standard non polar | 33892256 | | 7 alpha,26-Dihydroxy-4-cholesten-3-one,3TBDMS,isomer #1 | CC(CCC[C@@H](C)C1CCC2C3C(CC[C@@]21C)[C@@]1(C)CC=C(O[Si](C)(C)C(C)(C)C)C=C1C[C@H]3O[Si](C)(C)C(C)(C)C)CO[Si](C)(C)C(C)(C)C | 4238.7 | Standard non polar | 33892256 | | 7 alpha,26-Dihydroxy-4-cholesten-3-one,3TBDMS,isomer #1 | CC(CCC[C@@H](C)C1CCC2C3C(CC[C@@]21C)[C@@]1(C)CC=C(O[Si](C)(C)C(C)(C)C)C=C1C[C@H]3O[Si](C)(C)C(C)(C)C)CO[Si](C)(C)C(C)(C)C | 3905.8 | Standard polar | 33892256 |
| Show more...
|---|
| Spectra |
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - 7 alpha,26-Dihydroxy-4-cholesten-3-one GC-MS (Non-derivatized) - 70eV, Positive | splash10-0f79-0359200000-c5e766bc8da3445acdea | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 7 alpha,26-Dihydroxy-4-cholesten-3-one GC-MS (2 TMS) - 70eV, Positive | splash10-0002-1220390000-2ea93ad32d390af352da | 2017-10-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 7 alpha,26-Dihydroxy-4-cholesten-3-one GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 7 alpha,26-Dihydroxy-4-cholesten-3-one GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 7 alpha,26-Dihydroxy-4-cholesten-3-one 10V, Positive-QTOF | splash10-00kb-0009200000-f13064ffc70da2c2b57d | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 7 alpha,26-Dihydroxy-4-cholesten-3-one 20V, Positive-QTOF | splash10-00l2-1009000000-f344cdc48d7b49f22d6f | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 7 alpha,26-Dihydroxy-4-cholesten-3-one 40V, Positive-QTOF | splash10-016r-3129000000-b6c48651e7e9ac09edbc | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 7 alpha,26-Dihydroxy-4-cholesten-3-one 10V, Negative-QTOF | splash10-014i-0004900000-7754503899cd9db211c4 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 7 alpha,26-Dihydroxy-4-cholesten-3-one 20V, Negative-QTOF | splash10-014j-0009600000-54c6b067e6490ab83b85 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 7 alpha,26-Dihydroxy-4-cholesten-3-one 40V, Negative-QTOF | splash10-0674-2009000000-ceacf291ac96437f5944 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 7 alpha,26-Dihydroxy-4-cholesten-3-one 10V, Positive-QTOF | splash10-014j-0006900000-1bab66a6ddf2f8b577e8 | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 7 alpha,26-Dihydroxy-4-cholesten-3-one 20V, Positive-QTOF | splash10-07wj-5749100000-fb55e94ae37f95aa32a8 | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 7 alpha,26-Dihydroxy-4-cholesten-3-one 40V, Positive-QTOF | splash10-0a4i-9850000000-4478cbe8a6ea3e417fe2 | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 7 alpha,26-Dihydroxy-4-cholesten-3-one 10V, Negative-QTOF | splash10-014i-0000900000-67ea14fb7a26b9b2d929 | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 7 alpha,26-Dihydroxy-4-cholesten-3-one 20V, Negative-QTOF | splash10-014i-0005900000-37c9645751a2f52b7424 | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 7 alpha,26-Dihydroxy-4-cholesten-3-one 40V, Negative-QTOF | splash10-03di-0004900000-ca04c33318a7e2c042bc | 2021-09-22 | Wishart Lab | View Spectrum |
| Show more...
|---|
| Biological Properties |
|---|
| Cellular Locations | |
|---|
| Biospecimen Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
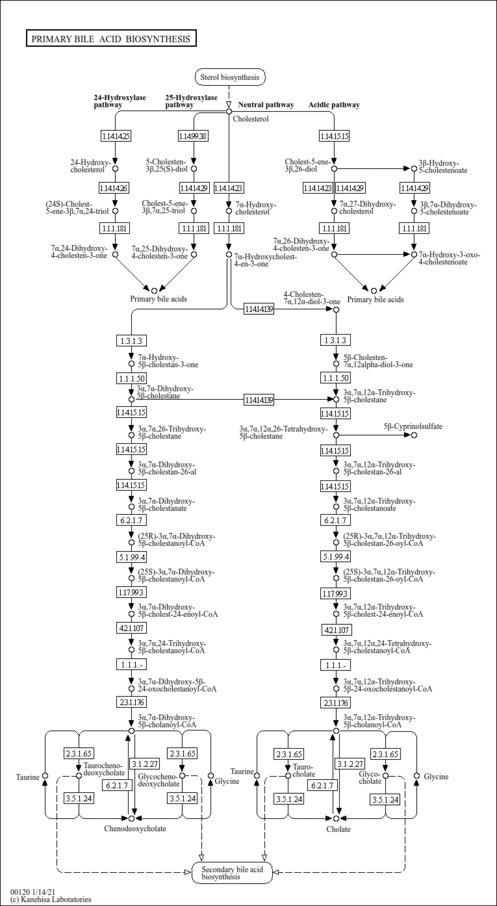
| Pathways | |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| Associated Disorders and Diseases |
|---|
| Disease References | None |
|---|
| Associated OMIM IDs | None |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| Phenol Explorer Compound ID | Not Available |
|---|
| FooDB ID | FDB029075 |
|---|
| KNApSAcK ID | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| KEGG Compound ID | C17336 |
|---|
| BioCyc ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PubChem Compound | 53481412 |
|---|
| PDB ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| Food Biomarker Ontology | Not Available |
|---|
| VMH ID | Not Available |
|---|
| MarkerDB ID | Not Available |
|---|
| Good Scents ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| General References | - Simons K, Toomre D: Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000 Oct;1(1):31-9. [PubMed:11413487 ]
- Watson AD: Thematic review series: systems biology approaches to metabolic and cardiovascular disorders. Lipidomics: a global approach to lipid analysis in biological systems. J Lipid Res. 2006 Oct;47(10):2101-11. Epub 2006 Aug 10. [PubMed:16902246 ]
- Sethi JK, Vidal-Puig AJ: Thematic review series: adipocyte biology. Adipose tissue function and plasticity orchestrate nutritional adaptation. J Lipid Res. 2007 Jun;48(6):1253-62. Epub 2007 Mar 20. [PubMed:17374880 ]
- Lingwood D, Simons K: Lipid rafts as a membrane-organizing principle. Science. 2010 Jan 1;327(5961):46-50. doi: 10.1126/science.1174621. [PubMed:20044567 ]
- Gunstone, Frank D., John L. Harwood, and Albert J. Dijkstra (2007). The lipid handbook with CD-ROM. CRC Press.
|
|---|