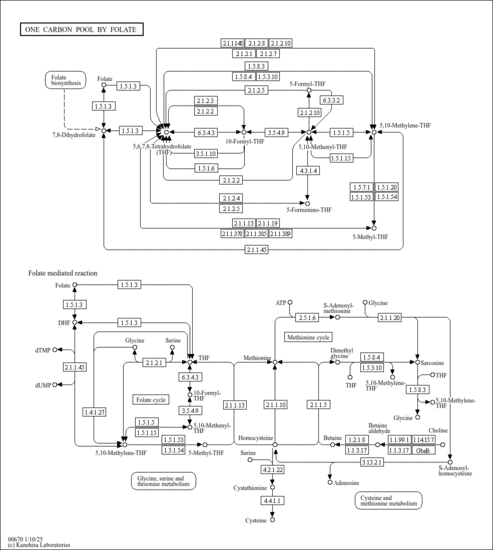
| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| 10-Formyltetrahydrofolate,1TMS,isomer #1 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N)[NH]3)C=C1)C(=O)O | 4973.5 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,1TMS,isomer #2 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N)[NH]3)C=C1 | 4953.5 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,1TMS,isomer #3 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C3)N2)[NH]1 | 5084.1 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,1TMS,isomer #4 | C[Si](C)(C)N1CC(CN(C=O)C2=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C2)NC2=C1[NH]C(N)=NC2=O | 4834.8 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,1TMS,isomer #5 | C[Si](C)(C)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N)[NH]3)C=C1)[C@@H](CCC(=O)O)C(=O)O | 4922.4 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,1TMS,isomer #6 | C[Si](C)(C)N1C2=C(NCC1CN(C=O)C1=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C1)[NH]C(N)=NC2=O | 4883.9 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,1TMS,isomer #7 | C[Si](C)(C)N1C(N)=NC(=O)C2=C1NCC(CN(C=O)C1=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C1)N2 | 4932.0 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,2TMS,isomer #1 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N)[NH]3)C=C1)C(=O)O[Si](C)(C)C | 4803.0 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,2TMS,isomer #10 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N)N3[Si](C)(C)C)C=C1 | 4793.6 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,2TMS,isomer #11 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N)[NH]3)N2[Si](C)(C)C)C=C1 | 4690.8 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,2TMS,isomer #12 | C[Si](C)(C)N(C1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C3)N2)[NH]1)[Si](C)(C)C | 4959.8 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,2TMS,isomer #13 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C3)N2)N1[Si](C)(C)C | 4965.8 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,2TMS,isomer #14 | C[Si](C)(C)NC1=NC(=O)C2=C([NH]1)N([Si](C)(C)C)CC(CN(C=O)C1=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C1)N2 | 4855.5 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,2TMS,isomer #15 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C3)N2)[NH]1 | 4908.4 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,2TMS,isomer #16 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C3)N2[Si](C)(C)C)[NH]1 | 4894.1 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,2TMS,isomer #17 | C[Si](C)(C)N1CC(CN(C=O)C2=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C2)NC2=C1[NH]C(N)=NC2=O | 4618.4 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,2TMS,isomer #18 | C[Si](C)(C)N1CC(CN(C=O)C2=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C2)N([Si](C)(C)C)C2=C1[NH]C(N)=NC2=O | 4615.4 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,2TMS,isomer #19 | C[Si](C)(C)N1CC(CN(C=O)C2=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C2)NC2=C1N([Si](C)(C)C)C(N)=NC2=O | 4716.4 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,2TMS,isomer #2 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C3)N2)[NH]1 | 4915.2 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,2TMS,isomer #20 | C[Si](C)(C)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N)N3[Si](C)(C)C)C=C1)[C@@H](CCC(=O)O)C(=O)O | 4743.9 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,2TMS,isomer #21 | C[Si](C)(C)N1C2=C(NCC1CN(C=O)C1=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1)[NH]C(N)=NC2=O | 4664.6 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,2TMS,isomer #22 | C[Si](C)(C)N1C2=C(NCC1CN(C=O)C1=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C1)N([Si](C)(C)C)C(N)=NC2=O | 4749.9 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,2TMS,isomer #3 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N)[NH]3)C=C1)[Si](C)(C)C | 4742.0 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,2TMS,isomer #4 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(N2)C(=O)N=C(N)[NH]3)C=C1)C(=O)O | 4665.9 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,2TMS,isomer #5 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N)N3[Si](C)(C)C)C=C1)C(=O)O | 4802.7 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,2TMS,isomer #6 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N)[NH]3)N2[Si](C)(C)C)C=C1)C(=O)O | 4684.6 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,2TMS,isomer #7 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C3)N2)[NH]1 | 4920.5 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,2TMS,isomer #8 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N)[NH]3)C=C1)[Si](C)(C)C | 4769.4 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,2TMS,isomer #9 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(N2)C(=O)N=C(N)[NH]3)C=C1 | 4665.3 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #1 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C3)N2)[NH]1 | 4769.3 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #1 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C3)N2)[NH]1 | 4263.2 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #1 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C3)N2)[NH]1 | 8009.6 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #10 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C3)N2[Si](C)(C)C)[NH]1 | 4713.3 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #10 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C3)N2[Si](C)(C)C)[NH]1 | 4342.8 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #10 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C3)N2[Si](C)(C)C)[NH]1 | 6763.5 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #11 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(N2)C(=O)N=C(N)[NH]3)C=C1)[Si](C)(C)C | 4463.8 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #11 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(N2)C(=O)N=C(N)[NH]3)C=C1)[Si](C)(C)C | 4210.2 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #11 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(N2)C(=O)N=C(N)[NH]3)C=C1)[Si](C)(C)C | 6922.0 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #12 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N)N3[Si](C)(C)C)C=C1)[Si](C)(C)C | 4591.1 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #12 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N)N3[Si](C)(C)C)C=C1)[Si](C)(C)C | 4149.2 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #12 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N)N3[Si](C)(C)C)C=C1)[Si](C)(C)C | 7188.8 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #13 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N)[NH]3)N2[Si](C)(C)C)C=C1)[Si](C)(C)C | 4489.7 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #13 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N)[NH]3)N2[Si](C)(C)C)C=C1)[Si](C)(C)C | 4189.2 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #13 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N)[NH]3)N2[Si](C)(C)C)C=C1)[Si](C)(C)C | 6561.4 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #14 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(N2)C(=O)N=C(N)N3[Si](C)(C)C)C=C1)C(=O)O | 4611.3 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #14 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(N2)C(=O)N=C(N)N3[Si](C)(C)C)C=C1)C(=O)O | 4283.0 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #14 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(N2)C(=O)N=C(N)N3[Si](C)(C)C)C=C1)C(=O)O | 7060.5 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #15 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(C(=O)N=C(N)[NH]3)N2[Si](C)(C)C)C=C1)C(=O)O | 4454.3 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #15 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(C(=O)N=C(N)[NH]3)N2[Si](C)(C)C)C=C1)C(=O)O | 4268.5 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #15 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(C(=O)N=C(N)[NH]3)N2[Si](C)(C)C)C=C1)C(=O)O | 6506.1 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #16 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N)N3[Si](C)(C)C)N2[Si](C)(C)C)C=C1)C(=O)O | 4607.6 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #16 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N)N3[Si](C)(C)C)N2[Si](C)(C)C)C=C1)C(=O)O | 4269.9 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #16 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N)N3[Si](C)(C)C)N2[Si](C)(C)C)C=C1)C(=O)O | 6819.9 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #17 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]3)C=C1 | 4772.3 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #17 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]3)C=C1 | 4334.1 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #17 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]3)C=C1 | 7690.3 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #18 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C3)N2)N1[Si](C)(C)C | 4809.1 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #18 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C3)N2)N1[Si](C)(C)C | 4300.8 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #18 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C3)N2)N1[Si](C)(C)C | 7709.4 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #19 | C[Si](C)(C)NC1=NC(=O)C2=C([NH]1)N([Si](C)(C)C)CC(CN(C=O)C1=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1)N2 | 4689.2 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #19 | C[Si](C)(C)NC1=NC(=O)C2=C([NH]1)N([Si](C)(C)C)CC(CN(C=O)C1=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1)N2 | 4336.3 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #19 | C[Si](C)(C)NC1=NC(=O)C2=C([NH]1)N([Si](C)(C)C)CC(CN(C=O)C1=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1)N2 | 7583.8 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #2 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O[Si](C)(C)C)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N)[NH]3)C=C1)[Si](C)(C)C | 4624.2 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #2 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O[Si](C)(C)C)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N)[NH]3)C=C1)[Si](C)(C)C | 4088.6 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #2 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O[Si](C)(C)C)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N)[NH]3)C=C1)[Si](C)(C)C | 7077.5 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #20 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C3)N2)[NH]1 | 4741.7 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #20 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C3)N2)[NH]1 | 4238.5 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #20 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C3)N2)[NH]1 | 7916.4 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #21 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C3)N2[Si](C)(C)C)[NH]1 | 4718.2 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #21 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C3)N2[Si](C)(C)C)[NH]1 | 4305.9 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #21 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C3)N2[Si](C)(C)C)[NH]1 | 6687.4 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #22 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(N2)C(=O)N=C(N)[NH]3)C=C1)[Si](C)(C)C | 4481.8 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #22 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(N2)C(=O)N=C(N)[NH]3)C=C1)[Si](C)(C)C | 4173.3 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #22 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(N2)C(=O)N=C(N)[NH]3)C=C1)[Si](C)(C)C | 6875.2 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #23 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N)N3[Si](C)(C)C)C=C1)[Si](C)(C)C | 4599.9 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #23 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N)N3[Si](C)(C)C)C=C1)[Si](C)(C)C | 4118.1 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #23 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N)N3[Si](C)(C)C)C=C1)[Si](C)(C)C | 7140.0 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #24 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N)[NH]3)N2[Si](C)(C)C)C=C1)[Si](C)(C)C | 4502.1 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #24 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N)[NH]3)N2[Si](C)(C)C)C=C1)[Si](C)(C)C | 4156.8 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #24 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N)[NH]3)N2[Si](C)(C)C)C=C1)[Si](C)(C)C | 6516.0 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #25 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(N2)C(=O)N=C(N)N3[Si](C)(C)C)C=C1 | 4594.7 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #25 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(N2)C(=O)N=C(N)N3[Si](C)(C)C)C=C1 | 4254.4 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #25 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(N2)C(=O)N=C(N)N3[Si](C)(C)C)C=C1 | 6982.6 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #26 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(C(=O)N=C(N)[NH]3)N2[Si](C)(C)C)C=C1 | 4444.8 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #26 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(C(=O)N=C(N)[NH]3)N2[Si](C)(C)C)C=C1 | 4221.0 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #26 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(C(=O)N=C(N)[NH]3)N2[Si](C)(C)C)C=C1 | 6433.4 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #27 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N)N3[Si](C)(C)C)N2[Si](C)(C)C)C=C1 | 4593.0 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #27 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N)N3[Si](C)(C)C)N2[Si](C)(C)C)C=C1 | 4236.9 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #27 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N)N3[Si](C)(C)C)N2[Si](C)(C)C)C=C1 | 6740.7 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #28 | C[Si](C)(C)N(C1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C3)N2)N1[Si](C)(C)C)[Si](C)(C)C | 4855.0 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #28 | C[Si](C)(C)N(C1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C3)N2)N1[Si](C)(C)C)[Si](C)(C)C | 4406.4 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #28 | C[Si](C)(C)N(C1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C3)N2)N1[Si](C)(C)C)[Si](C)(C)C | 7652.9 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #29 | C[Si](C)(C)N1CC(CN(C=O)C2=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C2)NC2=C1[NH]C(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O | 4726.2 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #29 | C[Si](C)(C)N1CC(CN(C=O)C2=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C2)NC2=C1[NH]C(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O | 4429.1 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #29 | C[Si](C)(C)N1CC(CN(C=O)C2=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C2)NC2=C1[NH]C(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O | 7478.9 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #3 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(N2)C(=O)N=C(N)[NH]3)C=C1)C(=O)O[Si](C)(C)C | 4516.7 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #3 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(N2)C(=O)N=C(N)[NH]3)C=C1)C(=O)O[Si](C)(C)C | 4199.0 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #3 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(N2)C(=O)N=C(N)[NH]3)C=C1)C(=O)O[Si](C)(C)C | 6959.9 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #30 | C[Si](C)(C)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]3)C=C1)[C@@H](CCC(=O)O)C(=O)O | 4729.8 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #30 | C[Si](C)(C)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]3)C=C1)[C@@H](CCC(=O)O)C(=O)O | 4335.4 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #30 | C[Si](C)(C)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]3)C=C1)[C@@H](CCC(=O)O)C(=O)O | 7667.8 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #31 | C[Si](C)(C)N1C2=C(NCC1CN(C=O)C1=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C1)[NH]C(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O | 4776.5 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #31 | C[Si](C)(C)N1C2=C(NCC1CN(C=O)C1=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C1)[NH]C(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O | 4479.8 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #31 | C[Si](C)(C)N1C2=C(NCC1CN(C=O)C1=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C1)[NH]C(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O | 6636.4 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #32 | C[Si](C)(C)NC1=NC(=O)C2=C(N([Si](C)(C)C)CC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C3)N2)N1[Si](C)(C)C | 4772.3 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #32 | C[Si](C)(C)NC1=NC(=O)C2=C(N([Si](C)(C)C)CC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C3)N2)N1[Si](C)(C)C | 4442.0 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #32 | C[Si](C)(C)NC1=NC(=O)C2=C(N([Si](C)(C)C)CC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C3)N2)N1[Si](C)(C)C | 7394.4 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #33 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C3)N2)N1[Si](C)(C)C | 4758.4 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #33 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C3)N2)N1[Si](C)(C)C | 4309.4 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #33 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C3)N2)N1[Si](C)(C)C | 7681.9 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #34 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C3)N2[Si](C)(C)C)N1[Si](C)(C)C | 4800.0 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #34 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C3)N2[Si](C)(C)C)N1[Si](C)(C)C | 4457.9 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #34 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C3)N2[Si](C)(C)C)N1[Si](C)(C)C | 6794.6 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #35 | C[Si](C)(C)NC1=NC(=O)C2=C([NH]1)N([Si](C)(C)C)CC(CN(C=O)C1=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1)N2 | 4648.5 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #35 | C[Si](C)(C)NC1=NC(=O)C2=C([NH]1)N([Si](C)(C)C)CC(CN(C=O)C1=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1)N2 | 4355.1 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #35 | C[Si](C)(C)NC1=NC(=O)C2=C([NH]1)N([Si](C)(C)C)CC(CN(C=O)C1=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1)N2 | 7547.1 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #36 | C[Si](C)(C)NC1=NC(=O)C2=C([NH]1)N([Si](C)(C)C)CC(CN(C=O)C1=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C1)N2[Si](C)(C)C | 4681.6 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #36 | C[Si](C)(C)NC1=NC(=O)C2=C([NH]1)N([Si](C)(C)C)CC(CN(C=O)C1=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C1)N2[Si](C)(C)C | 4405.0 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #36 | C[Si](C)(C)NC1=NC(=O)C2=C([NH]1)N([Si](C)(C)C)CC(CN(C=O)C1=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C1)N2[Si](C)(C)C | 6470.1 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #37 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C3)N2[Si](C)(C)C)[NH]1 | 4698.8 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #37 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C3)N2[Si](C)(C)C)[NH]1 | 4343.0 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #37 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C3)N2[Si](C)(C)C)[NH]1 | 6720.1 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #38 | C[Si](C)(C)N1CC(CN(C=O)C2=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C2)N([Si](C)(C)C)C2=C1[NH]C(N)=NC2=O | 4406.4 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #38 | C[Si](C)(C)N1CC(CN(C=O)C2=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C2)N([Si](C)(C)C)C2=C1[NH]C(N)=NC2=O | 4250.6 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #38 | C[Si](C)(C)N1CC(CN(C=O)C2=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C2)N([Si](C)(C)C)C2=C1[NH]C(N)=NC2=O | 6423.8 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #39 | C[Si](C)(C)N1CC(CN(C=O)C2=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C2)NC2=C1N([Si](C)(C)C)C(N)=NC2=O | 4537.5 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #39 | C[Si](C)(C)N1CC(CN(C=O)C2=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C2)NC2=C1N([Si](C)(C)C)C(N)=NC2=O | 4260.0 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #39 | C[Si](C)(C)N1CC(CN(C=O)C2=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C2)NC2=C1N([Si](C)(C)C)C(N)=NC2=O | 6971.6 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #4 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N)N3[Si](C)(C)C)C=C1)C(=O)O[Si](C)(C)C | 4647.4 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #4 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N)N3[Si](C)(C)C)C=C1)C(=O)O[Si](C)(C)C | 4140.3 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #4 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N)N3[Si](C)(C)C)C=C1)C(=O)O[Si](C)(C)C | 7211.5 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #40 | C[Si](C)(C)N1CC(CN(C=O)C2=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C2)N([Si](C)(C)C)C2=C1N([Si](C)(C)C)C(N)=NC2=O | 4591.4 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #40 | C[Si](C)(C)N1CC(CN(C=O)C2=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C2)N([Si](C)(C)C)C2=C1N([Si](C)(C)C)C(N)=NC2=O | 4366.8 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #40 | C[Si](C)(C)N1CC(CN(C=O)C2=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C2)N([Si](C)(C)C)C2=C1N([Si](C)(C)C)C(N)=NC2=O | 6615.7 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #41 | C[Si](C)(C)N1C2=C(NCC1CN(C=O)C1=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1)N([Si](C)(C)C)C(N)=NC2=O | 4553.5 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #41 | C[Si](C)(C)N1C2=C(NCC1CN(C=O)C1=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1)N([Si](C)(C)C)C(N)=NC2=O | 4292.9 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #41 | C[Si](C)(C)N1C2=C(NCC1CN(C=O)C1=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1)N([Si](C)(C)C)C(N)=NC2=O | 6784.5 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #5 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N)[NH]3)N2[Si](C)(C)C)C=C1)C(=O)O[Si](C)(C)C | 4547.5 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #5 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N)[NH]3)N2[Si](C)(C)C)C=C1)C(=O)O[Si](C)(C)C | 4153.4 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #5 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N)[NH]3)N2[Si](C)(C)C)C=C1)C(=O)O[Si](C)(C)C | 6576.6 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #6 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]3)C=C1)C(=O)O | 4758.5 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #6 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]3)C=C1)C(=O)O | 4372.8 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #6 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]3)C=C1)C(=O)O | 7769.8 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #7 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C3)N2)N1[Si](C)(C)C | 4817.6 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #7 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C3)N2)N1[Si](C)(C)C | 4328.3 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #7 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C3)N2)N1[Si](C)(C)C | 7786.0 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #8 | C[Si](C)(C)NC1=NC(=O)C2=C([NH]1)N([Si](C)(C)C)CC(CN(C=O)C1=CC=C(C(=O)N[C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1)N2 | 4697.2 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #8 | C[Si](C)(C)NC1=NC(=O)C2=C([NH]1)N([Si](C)(C)C)CC(CN(C=O)C1=CC=C(C(=O)N[C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1)N2 | 4363.4 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #8 | C[Si](C)(C)NC1=NC(=O)C2=C([NH]1)N([Si](C)(C)C)CC(CN(C=O)C1=CC=C(C(=O)N[C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1)N2 | 7655.2 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #9 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C3)N2)[NH]1 | 4716.5 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #9 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C3)N2)[NH]1 | 4270.1 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TMS,isomer #9 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C3)N2)[NH]1 | 7964.0 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #1 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]3)C=C1)C(=O)O[Si](C)(C)C | 4633.8 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #1 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]3)C=C1)C(=O)O[Si](C)(C)C | 4304.9 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #1 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]3)C=C1)C(=O)O[Si](C)(C)C | 7344.8 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #10 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(C(=O)N=C(N)[NH]3)N2[Si](C)(C)C)C=C1)C(=O)O[Si](C)(C)C | 4307.1 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #10 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(C(=O)N=C(N)[NH]3)N2[Si](C)(C)C)C=C1)C(=O)O[Si](C)(C)C | 4149.8 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #10 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(C(=O)N=C(N)[NH]3)N2[Si](C)(C)C)C=C1)C(=O)O[Si](C)(C)C | 6187.9 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #11 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N)N3[Si](C)(C)C)N2[Si](C)(C)C)C=C1)C(=O)O[Si](C)(C)C | 4494.7 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #11 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N)N3[Si](C)(C)C)N2[Si](C)(C)C)C=C1)C(=O)O[Si](C)(C)C | 4147.4 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #11 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N)N3[Si](C)(C)C)N2[Si](C)(C)C)C=C1)C(=O)O[Si](C)(C)C | 6461.4 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #12 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]3)C=C1)[Si](C)(C)C | 4566.1 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #12 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]3)C=C1)[Si](C)(C)C | 4317.0 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #12 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]3)C=C1)[Si](C)(C)C | 7302.8 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #13 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(N2)C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]3)C=C1)C(=O)O | 4590.3 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #13 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(N2)C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]3)C=C1)C(=O)O | 4411.2 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #13 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(N2)C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]3)C=C1)C(=O)O | 7168.4 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #14 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)N3[Si](C)(C)C)C=C1)C(=O)O | 4711.5 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #14 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)N3[Si](C)(C)C)C=C1)C(=O)O | 4366.9 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #14 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)N3[Si](C)(C)C)C=C1)C(=O)O | 7160.4 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #15 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]3)N2[Si](C)(C)C)C=C1)C(=O)O | 4622.8 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #15 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]3)N2[Si](C)(C)C)C=C1)C(=O)O | 4352.8 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #15 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]3)N2[Si](C)(C)C)C=C1)C(=O)O | 6207.9 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #16 | C[Si](C)(C)NC1=NC(=O)C2=C(N([Si](C)(C)C)CC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C3)N2)N1[Si](C)(C)C | 4642.0 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #16 | C[Si](C)(C)NC1=NC(=O)C2=C(N([Si](C)(C)C)CC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C3)N2)N1[Si](C)(C)C | 4405.7 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #16 | C[Si](C)(C)NC1=NC(=O)C2=C(N([Si](C)(C)C)CC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C3)N2)N1[Si](C)(C)C | 7077.9 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #17 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C3)N2)N1[Si](C)(C)C | 4609.0 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #17 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C3)N2)N1[Si](C)(C)C | 4255.7 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #17 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C3)N2)N1[Si](C)(C)C | 7343.7 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #18 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C3)N2[Si](C)(C)C)N1[Si](C)(C)C | 4676.1 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #18 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C3)N2[Si](C)(C)C)N1[Si](C)(C)C | 4331.2 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #18 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C3)N2[Si](C)(C)C)N1[Si](C)(C)C | 6421.6 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #19 | C[Si](C)(C)NC1=NC(=O)C2=C([NH]1)N([Si](C)(C)C)CC(CN(C=O)C1=CC=C(C(=O)N([C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1)N2 | 4504.7 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #19 | C[Si](C)(C)NC1=NC(=O)C2=C([NH]1)N([Si](C)(C)C)CC(CN(C=O)C1=CC=C(C(=O)N([C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1)N2 | 4318.3 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #19 | C[Si](C)(C)NC1=NC(=O)C2=C([NH]1)N([Si](C)(C)C)CC(CN(C=O)C1=CC=C(C(=O)N([C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1)N2 | 7251.7 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #2 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C3)N2)N1[Si](C)(C)C | 4681.7 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #2 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C3)N2)N1[Si](C)(C)C | 4231.5 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #2 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C3)N2)N1[Si](C)(C)C | 7385.6 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #20 | C[Si](C)(C)NC1=NC(=O)C2=C([NH]1)N([Si](C)(C)C)CC(CN(C=O)C1=CC=C(C(=O)N[C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1)N2[Si](C)(C)C | 4543.5 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #20 | C[Si](C)(C)NC1=NC(=O)C2=C([NH]1)N([Si](C)(C)C)CC(CN(C=O)C1=CC=C(C(=O)N[C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1)N2[Si](C)(C)C | 4300.7 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #20 | C[Si](C)(C)NC1=NC(=O)C2=C([NH]1)N([Si](C)(C)C)CC(CN(C=O)C1=CC=C(C(=O)N[C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1)N2[Si](C)(C)C | 6123.6 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #21 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C3)N2[Si](C)(C)C)[NH]1 | 4546.2 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #21 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C3)N2[Si](C)(C)C)[NH]1 | 4245.9 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #21 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C3)N2[Si](C)(C)C)[NH]1 | 6342.3 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #22 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(N2)C(=O)N=C(N)N3[Si](C)(C)C)C=C1)[Si](C)(C)C | 4417.5 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #22 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(N2)C(=O)N=C(N)N3[Si](C)(C)C)C=C1)[Si](C)(C)C | 4258.5 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #22 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(N2)C(=O)N=C(N)N3[Si](C)(C)C)C=C1)[Si](C)(C)C | 6679.2 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #23 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(C(=O)N=C(N)[NH]3)N2[Si](C)(C)C)C=C1)[Si](C)(C)C | 4287.2 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #23 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(C(=O)N=C(N)[NH]3)N2[Si](C)(C)C)C=C1)[Si](C)(C)C | 4185.2 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #23 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(C(=O)N=C(N)[NH]3)N2[Si](C)(C)C)C=C1)[Si](C)(C)C | 6161.1 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #24 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N)N3[Si](C)(C)C)N2[Si](C)(C)C)C=C1)[Si](C)(C)C | 4438.8 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #24 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N)N3[Si](C)(C)C)N2[Si](C)(C)C)C=C1)[Si](C)(C)C | 4192.9 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #24 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N)N3[Si](C)(C)C)N2[Si](C)(C)C)C=C1)[Si](C)(C)C | 6444.0 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #25 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(C(=O)N=C(N)N3[Si](C)(C)C)N2[Si](C)(C)C)C=C1)C(=O)O | 4475.9 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #25 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(C(=O)N=C(N)N3[Si](C)(C)C)N2[Si](C)(C)C)C=C1)C(=O)O | 4287.1 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #25 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(C(=O)N=C(N)N3[Si](C)(C)C)N2[Si](C)(C)C)C=C1)C(=O)O | 6347.5 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #26 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]3)C=C1)[Si](C)(C)C | 4592.4 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #26 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]3)C=C1)[Si](C)(C)C | 4293.0 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #26 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]3)C=C1)[Si](C)(C)C | 7260.9 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #27 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(N2)C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]3)C=C1 | 4587.1 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #27 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(N2)C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]3)C=C1 | 4391.5 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #27 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(N2)C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]3)C=C1 | 7107.0 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #28 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)N3[Si](C)(C)C)C=C1 | 4699.2 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #28 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)N3[Si](C)(C)C)C=C1 | 4346.8 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #28 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)N3[Si](C)(C)C)C=C1 | 7095.2 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #29 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]3)N2[Si](C)(C)C)C=C1 | 4625.3 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #29 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]3)N2[Si](C)(C)C)C=C1 | 4329.9 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #29 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]3)N2[Si](C)(C)C)C=C1 | 6144.8 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #3 | C[Si](C)(C)NC1=NC(=O)C2=C([NH]1)N([Si](C)(C)C)CC(CN(C=O)C1=CC=C(C(=O)N[C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1)N2 | 4547.3 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #3 | C[Si](C)(C)NC1=NC(=O)C2=C([NH]1)N([Si](C)(C)C)CC(CN(C=O)C1=CC=C(C(=O)N[C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1)N2 | 4285.3 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #3 | C[Si](C)(C)NC1=NC(=O)C2=C([NH]1)N([Si](C)(C)C)CC(CN(C=O)C1=CC=C(C(=O)N[C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1)N2 | 7286.9 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #30 | C[Si](C)(C)NC1=NC(=O)C2=C(N([Si](C)(C)C)CC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C3)N2)N1[Si](C)(C)C | 4647.6 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #30 | C[Si](C)(C)NC1=NC(=O)C2=C(N([Si](C)(C)C)CC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C3)N2)N1[Si](C)(C)C | 4388.4 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #30 | C[Si](C)(C)NC1=NC(=O)C2=C(N([Si](C)(C)C)CC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C3)N2)N1[Si](C)(C)C | 7017.7 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #31 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C3)N2)N1[Si](C)(C)C | 4624.9 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #31 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C3)N2)N1[Si](C)(C)C | 4239.4 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #31 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C3)N2)N1[Si](C)(C)C | 7303.3 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #32 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C3)N2[Si](C)(C)C)N1[Si](C)(C)C | 4670.2 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #32 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C3)N2[Si](C)(C)C)N1[Si](C)(C)C | 4319.1 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #32 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C3)N2[Si](C)(C)C)N1[Si](C)(C)C | 6360.4 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #33 | C[Si](C)(C)NC1=NC(=O)C2=C([NH]1)N([Si](C)(C)C)CC(CN(C=O)C1=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)N2 | 4515.6 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #33 | C[Si](C)(C)NC1=NC(=O)C2=C([NH]1)N([Si](C)(C)C)CC(CN(C=O)C1=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)N2 | 4297.8 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #33 | C[Si](C)(C)NC1=NC(=O)C2=C([NH]1)N([Si](C)(C)C)CC(CN(C=O)C1=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)N2 | 7214.1 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #34 | C[Si](C)(C)NC1=NC(=O)C2=C([NH]1)N([Si](C)(C)C)CC(CN(C=O)C1=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1)N2[Si](C)(C)C | 4541.9 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #34 | C[Si](C)(C)NC1=NC(=O)C2=C([NH]1)N([Si](C)(C)C)CC(CN(C=O)C1=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1)N2[Si](C)(C)C | 4275.2 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #34 | C[Si](C)(C)NC1=NC(=O)C2=C([NH]1)N([Si](C)(C)C)CC(CN(C=O)C1=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1)N2[Si](C)(C)C | 6067.6 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #35 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C3)N2[Si](C)(C)C)[NH]1 | 4562.2 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #35 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C3)N2[Si](C)(C)C)[NH]1 | 4231.0 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #35 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C3)N2[Si](C)(C)C)[NH]1 | 6306.6 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #36 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(N2)C(=O)N=C(N)N3[Si](C)(C)C)C=C1)[Si](C)(C)C | 4434.5 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #36 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(N2)C(=O)N=C(N)N3[Si](C)(C)C)C=C1)[Si](C)(C)C | 4235.1 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #36 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(N2)C(=O)N=C(N)N3[Si](C)(C)C)C=C1)[Si](C)(C)C | 6641.5 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #37 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(C(=O)N=C(N)[NH]3)N2[Si](C)(C)C)C=C1)[Si](C)(C)C | 4291.0 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #37 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(C(=O)N=C(N)[NH]3)N2[Si](C)(C)C)C=C1)[Si](C)(C)C | 4162.3 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #37 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(C(=O)N=C(N)[NH]3)N2[Si](C)(C)C)C=C1)[Si](C)(C)C | 6127.4 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #38 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N)N3[Si](C)(C)C)N2[Si](C)(C)C)C=C1)[Si](C)(C)C | 4451.5 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #38 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N)N3[Si](C)(C)C)N2[Si](C)(C)C)C=C1)[Si](C)(C)C | 4175.8 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #38 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N)N3[Si](C)(C)C)N2[Si](C)(C)C)C=C1)[Si](C)(C)C | 6406.2 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #39 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(C(=O)N=C(N)N3[Si](C)(C)C)N2[Si](C)(C)C)C=C1 | 4462.3 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #39 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(C(=O)N=C(N)N3[Si](C)(C)C)N2[Si](C)(C)C)C=C1 | 4260.8 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #39 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(C(=O)N=C(N)N3[Si](C)(C)C)N2[Si](C)(C)C)C=C1 | 6285.5 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #4 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C3)N2)[NH]1 | 4598.3 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #4 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C3)N2)[NH]1 | 4193.4 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #4 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C3)N2)[NH]1 | 7562.6 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #40 | C[Si](C)(C)N1CC(CN(C=O)C2=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C2)NC2=C1N([Si](C)(C)C)C(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O | 4679.8 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #40 | C[Si](C)(C)N1CC(CN(C=O)C2=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C2)NC2=C1N([Si](C)(C)C)C(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O | 4494.9 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #40 | C[Si](C)(C)N1CC(CN(C=O)C2=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C2)NC2=C1N([Si](C)(C)C)C(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O | 6902.6 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #41 | C[Si](C)(C)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)N3[Si](C)(C)C)C=C1)[C@@H](CCC(=O)O)C(=O)O | 4638.6 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #41 | C[Si](C)(C)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)N3[Si](C)(C)C)C=C1)[C@@H](CCC(=O)O)C(=O)O | 4361.3 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #41 | C[Si](C)(C)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)N3[Si](C)(C)C)C=C1)[C@@H](CCC(=O)O)C(=O)O | 7065.1 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #42 | C[Si](C)(C)N1C2=C(NCC1CN(C=O)C1=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C1)N([Si](C)(C)C)C(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O | 4740.3 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #42 | C[Si](C)(C)N1C2=C(NCC1CN(C=O)C1=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C1)N([Si](C)(C)C)C(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O | 4481.6 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #42 | C[Si](C)(C)N1C2=C(NCC1CN(C=O)C1=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C1)N([Si](C)(C)C)C(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O | 6297.8 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #43 | C[Si](C)(C)N1CC(CN(C=O)C2=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C2)NC2=C1[NH]C(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O | 4522.8 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #43 | C[Si](C)(C)N1CC(CN(C=O)C2=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C2)NC2=C1[NH]C(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O | 4412.3 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #43 | C[Si](C)(C)N1CC(CN(C=O)C2=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C2)NC2=C1[NH]C(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O | 7064.9 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #44 | C[Si](C)(C)N1CC(CN(C=O)C2=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C2)N([Si](C)(C)C)C2=C1[NH]C(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O | 4567.9 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #44 | C[Si](C)(C)N1CC(CN(C=O)C2=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C2)N([Si](C)(C)C)C2=C1[NH]C(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O | 4435.4 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #44 | C[Si](C)(C)N1CC(CN(C=O)C2=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C2)N([Si](C)(C)C)C2=C1[NH]C(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O | 6009.4 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #45 | C[Si](C)(C)N1C2=C(NCC1CN(C=O)C1=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1)[NH]C(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O | 4577.1 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #45 | C[Si](C)(C)N1C2=C(NCC1CN(C=O)C1=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1)[NH]C(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O | 4399.8 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #45 | C[Si](C)(C)N1C2=C(NCC1CN(C=O)C1=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1)[NH]C(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O | 6215.3 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #46 | C[Si](C)(C)NC1=NC(=O)C2=C(N([Si](C)(C)C)CC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C3)N2)N1[Si](C)(C)C | 4584.1 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #46 | C[Si](C)(C)NC1=NC(=O)C2=C(N([Si](C)(C)C)CC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C3)N2)N1[Si](C)(C)C | 4409.3 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #46 | C[Si](C)(C)NC1=NC(=O)C2=C(N([Si](C)(C)C)CC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C3)N2)N1[Si](C)(C)C | 6967.8 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #47 | C[Si](C)(C)NC1=NC(=O)C2=C(N([Si](C)(C)C)CC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C3)N2[Si](C)(C)C)N1[Si](C)(C)C | 4655.8 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #47 | C[Si](C)(C)NC1=NC(=O)C2=C(N([Si](C)(C)C)CC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C3)N2[Si](C)(C)C)N1[Si](C)(C)C | 4438.7 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #47 | C[Si](C)(C)NC1=NC(=O)C2=C(N([Si](C)(C)C)CC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C3)N2[Si](C)(C)C)N1[Si](C)(C)C | 6075.5 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #48 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C3)N2[Si](C)(C)C)N1[Si](C)(C)C | 4624.5 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #48 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C3)N2[Si](C)(C)C)N1[Si](C)(C)C | 4357.1 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #48 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C3)N2[Si](C)(C)C)N1[Si](C)(C)C | 6385.7 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #49 | C[Si](C)(C)NC1=NC(=O)C2=C([NH]1)N([Si](C)(C)C)CC(CN(C=O)C1=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1)N2[Si](C)(C)C | 4517.2 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #49 | C[Si](C)(C)NC1=NC(=O)C2=C([NH]1)N([Si](C)(C)C)CC(CN(C=O)C1=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1)N2[Si](C)(C)C | 4310.5 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #49 | C[Si](C)(C)NC1=NC(=O)C2=C([NH]1)N([Si](C)(C)C)CC(CN(C=O)C1=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1)N2[Si](C)(C)C | 6090.4 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #5 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C3)N2[Si](C)(C)C)[NH]1 | 4581.1 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #5 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C3)N2[Si](C)(C)C)[NH]1 | 4193.5 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #5 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C3)N2[Si](C)(C)C)[NH]1 | 6306.8 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #50 | C[Si](C)(C)N1CC(CN(C=O)C2=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C2)N([Si](C)(C)C)C2=C1N([Si](C)(C)C)C(N)=NC2=O | 4419.1 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #50 | C[Si](C)(C)N1CC(CN(C=O)C2=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C2)N([Si](C)(C)C)C2=C1N([Si](C)(C)C)C(N)=NC2=O | 4281.7 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #50 | C[Si](C)(C)N1CC(CN(C=O)C2=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C2)N([Si](C)(C)C)C2=C1N([Si](C)(C)C)C(N)=NC2=O | 6276.2 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #6 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O[Si](C)(C)C)N(C(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(N2)C(=O)N=C(N)[NH]3)C=C1)[Si](C)(C)C | 4339.8 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #6 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O[Si](C)(C)C)N(C(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(N2)C(=O)N=C(N)[NH]3)C=C1)[Si](C)(C)C | 4156.4 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #6 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O[Si](C)(C)C)N(C(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(N2)C(=O)N=C(N)[NH]3)C=C1)[Si](C)(C)C | 6599.2 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #7 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O[Si](C)(C)C)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N)N3[Si](C)(C)C)C=C1)[Si](C)(C)C | 4498.1 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #7 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O[Si](C)(C)C)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N)N3[Si](C)(C)C)C=C1)[Si](C)(C)C | 4094.7 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #7 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O[Si](C)(C)C)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N)N3[Si](C)(C)C)C=C1)[Si](C)(C)C | 6825.2 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #8 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O[Si](C)(C)C)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N)[NH]3)N2[Si](C)(C)C)C=C1)[Si](C)(C)C | 4389.7 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #8 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O[Si](C)(C)C)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N)[NH]3)N2[Si](C)(C)C)C=C1)[Si](C)(C)C | 4104.7 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #8 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O[Si](C)(C)C)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N)[NH]3)N2[Si](C)(C)C)C=C1)[Si](C)(C)C | 6228.3 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #9 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(N2)C(=O)N=C(N)N3[Si](C)(C)C)C=C1)C(=O)O[Si](C)(C)C | 4465.9 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #9 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(N2)C(=O)N=C(N)N3[Si](C)(C)C)C=C1)C(=O)O[Si](C)(C)C | 4237.7 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,4TMS,isomer #9 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(N2)C(=O)N=C(N)N3[Si](C)(C)C)C=C1)C(=O)O[Si](C)(C)C | 6716.2 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #1 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O[Si](C)(C)C)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]3)C=C1)[Si](C)(C)C | 4494.3 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #1 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O[Si](C)(C)C)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]3)C=C1)[Si](C)(C)C | 4261.3 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #1 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O[Si](C)(C)C)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]3)C=C1)[Si](C)(C)C | 6964.3 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #10 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C3)N2[Si](C)(C)C)[NH]1 | 4466.1 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #10 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C3)N2[Si](C)(C)C)[NH]1 | 4147.3 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #10 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C3)N2[Si](C)(C)C)[NH]1 | 5955.3 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #11 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O[Si](C)(C)C)N(C(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(N2)C(=O)N=C(N)N3[Si](C)(C)C)C=C1)[Si](C)(C)C | 4340.0 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #11 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O[Si](C)(C)C)N(C(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(N2)C(=O)N=C(N)N3[Si](C)(C)C)C=C1)[Si](C)(C)C | 4215.8 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #11 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O[Si](C)(C)C)N(C(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(N2)C(=O)N=C(N)N3[Si](C)(C)C)C=C1)[Si](C)(C)C | 6414.6 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #12 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O[Si](C)(C)C)N(C(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(C(=O)N=C(N)[NH]3)N2[Si](C)(C)C)C=C1)[Si](C)(C)C | 4210.9 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #12 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O[Si](C)(C)C)N(C(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(C(=O)N=C(N)[NH]3)N2[Si](C)(C)C)C=C1)[Si](C)(C)C | 4110.3 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #12 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O[Si](C)(C)C)N(C(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(C(=O)N=C(N)[NH]3)N2[Si](C)(C)C)C=C1)[Si](C)(C)C | 5912.5 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #13 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O[Si](C)(C)C)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N)N3[Si](C)(C)C)N2[Si](C)(C)C)C=C1)[Si](C)(C)C | 4393.0 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #13 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O[Si](C)(C)C)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N)N3[Si](C)(C)C)N2[Si](C)(C)C)C=C1)[Si](C)(C)C | 4113.5 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #13 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O[Si](C)(C)C)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N)N3[Si](C)(C)C)N2[Si](C)(C)C)C=C1)[Si](C)(C)C | 6171.9 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #14 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(C(=O)N=C(N)N3[Si](C)(C)C)N2[Si](C)(C)C)C=C1)C(=O)O[Si](C)(C)C | 4376.5 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #14 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(C(=O)N=C(N)N3[Si](C)(C)C)N2[Si](C)(C)C)C=C1)C(=O)O[Si](C)(C)C | 4188.7 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #14 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(C(=O)N=C(N)N3[Si](C)(C)C)N2[Si](C)(C)C)C=C1)C(=O)O[Si](C)(C)C | 6076.5 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #15 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(N2)C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]3)C=C1)[Si](C)(C)C | 4411.1 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #15 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(N2)C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]3)C=C1)[Si](C)(C)C | 4381.5 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #15 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(N2)C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]3)C=C1)[Si](C)(C)C | 6794.9 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #16 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)N3[Si](C)(C)C)C=C1)[Si](C)(C)C | 4533.4 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #16 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)N3[Si](C)(C)C)C=C1)[Si](C)(C)C | 4310.3 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #16 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)N3[Si](C)(C)C)C=C1)[Si](C)(C)C | 6754.6 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #17 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]3)N2[Si](C)(C)C)C=C1)[Si](C)(C)C | 4469.1 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #17 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]3)N2[Si](C)(C)C)C=C1)[Si](C)(C)C | 4296.0 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #17 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]3)N2[Si](C)(C)C)C=C1)[Si](C)(C)C | 5836.5 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #18 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(N2)C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)N3[Si](C)(C)C)C=C1)C(=O)O | 4582.6 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #18 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(N2)C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)N3[Si](C)(C)C)C=C1)C(=O)O | 4458.8 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #18 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(N2)C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)N3[Si](C)(C)C)C=C1)C(=O)O | 6605.2 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #19 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]3)N2[Si](C)(C)C)C=C1)C(=O)O | 4489.2 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #19 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]3)N2[Si](C)(C)C)C=C1)C(=O)O | 4347.1 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #19 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]3)N2[Si](C)(C)C)C=C1)C(=O)O | 5696.5 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #2 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(N2)C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]3)C=C1)C(=O)O[Si](C)(C)C | 4486.0 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #2 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(N2)C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]3)C=C1)C(=O)O[Si](C)(C)C | 4344.1 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #2 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(N2)C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]3)C=C1)C(=O)O[Si](C)(C)C | 6837.7 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #20 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)N3[Si](C)(C)C)N2[Si](C)(C)C)C=C1)C(=O)O | 4621.0 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #20 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)N3[Si](C)(C)C)N2[Si](C)(C)C)C=C1)C(=O)O | 4331.6 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #20 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)N3[Si](C)(C)C)N2[Si](C)(C)C)C=C1)C(=O)O | 5920.9 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #21 | C[Si](C)(C)NC1=NC(=O)C2=C(N([Si](C)(C)C)CC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C3)N2)N1[Si](C)(C)C | 4462.8 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #21 | C[Si](C)(C)NC1=NC(=O)C2=C(N([Si](C)(C)C)CC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C3)N2)N1[Si](C)(C)C | 4362.3 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #21 | C[Si](C)(C)NC1=NC(=O)C2=C(N([Si](C)(C)C)CC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C3)N2)N1[Si](C)(C)C | 6691.8 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #22 | C[Si](C)(C)NC1=NC(=O)C2=C(N([Si](C)(C)C)CC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C3)N2[Si](C)(C)C)N1[Si](C)(C)C | 4542.5 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #22 | C[Si](C)(C)NC1=NC(=O)C2=C(N([Si](C)(C)C)CC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C3)N2[Si](C)(C)C)N1[Si](C)(C)C | 4325.4 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #22 | C[Si](C)(C)NC1=NC(=O)C2=C(N([Si](C)(C)C)CC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C3)N2[Si](C)(C)C)N1[Si](C)(C)C | 5768.2 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #23 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C3)N2[Si](C)(C)C)N1[Si](C)(C)C | 4511.1 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #23 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C3)N2[Si](C)(C)C)N1[Si](C)(C)C | 4257.0 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #23 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C3)N2[Si](C)(C)C)N1[Si](C)(C)C | 6062.0 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #24 | C[Si](C)(C)NC1=NC(=O)C2=C([NH]1)N([Si](C)(C)C)CC(CN(C=O)C1=CC=C(C(=O)N([C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1)N2[Si](C)(C)C | 4409.1 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #24 | C[Si](C)(C)NC1=NC(=O)C2=C([NH]1)N([Si](C)(C)C)CC(CN(C=O)C1=CC=C(C(=O)N([C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1)N2[Si](C)(C)C | 4236.5 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #24 | C[Si](C)(C)NC1=NC(=O)C2=C([NH]1)N([Si](C)(C)C)CC(CN(C=O)C1=CC=C(C(=O)N([C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1)N2[Si](C)(C)C | 5787.2 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #25 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(C(=O)N=C(N)N3[Si](C)(C)C)N2[Si](C)(C)C)C=C1)[Si](C)(C)C | 4336.6 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #25 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(C(=O)N=C(N)N3[Si](C)(C)C)N2[Si](C)(C)C)C=C1)[Si](C)(C)C | 4224.4 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #25 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(C(=O)N=C(N)N3[Si](C)(C)C)N2[Si](C)(C)C)C=C1)[Si](C)(C)C | 6057.7 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #26 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(N2)C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]3)C=C1)[Si](C)(C)C | 4419.7 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #26 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(N2)C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]3)C=C1)[Si](C)(C)C | 4365.3 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #26 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(N2)C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]3)C=C1)[Si](C)(C)C | 6766.3 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #27 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)N3[Si](C)(C)C)C=C1)[Si](C)(C)C | 4541.3 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #27 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)N3[Si](C)(C)C)C=C1)[Si](C)(C)C | 4299.6 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #27 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)N3[Si](C)(C)C)C=C1)[Si](C)(C)C | 6724.7 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #28 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]3)N2[Si](C)(C)C)C=C1)[Si](C)(C)C | 4483.1 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #28 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]3)N2[Si](C)(C)C)C=C1)[Si](C)(C)C | 4286.6 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #28 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]3)N2[Si](C)(C)C)C=C1)[Si](C)(C)C | 5807.9 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #29 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(N2)C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)N3[Si](C)(C)C)C=C1 | 4581.2 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #29 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(N2)C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)N3[Si](C)(C)C)C=C1 | 4446.6 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #29 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(N2)C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)N3[Si](C)(C)C)C=C1 | 6556.2 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #3 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)N3[Si](C)(C)C)C=C1)C(=O)O[Si](C)(C)C | 4604.6 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #3 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)N3[Si](C)(C)C)C=C1)C(=O)O[Si](C)(C)C | 4278.3 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #3 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)N3[Si](C)(C)C)C=C1)C(=O)O[Si](C)(C)C | 6799.2 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #30 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]3)N2[Si](C)(C)C)C=C1 | 4479.0 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #30 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]3)N2[Si](C)(C)C)C=C1 | 4331.7 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #30 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]3)N2[Si](C)(C)C)C=C1 | 5652.4 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #31 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)N3[Si](C)(C)C)N2[Si](C)(C)C)C=C1 | 4616.9 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #31 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)N3[Si](C)(C)C)N2[Si](C)(C)C)C=C1 | 4329.4 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #31 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)N3[Si](C)(C)C)N2[Si](C)(C)C)C=C1 | 5872.7 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #32 | C[Si](C)(C)NC1=NC(=O)C2=C(N([Si](C)(C)C)CC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C3)N2)N1[Si](C)(C)C | 4476.2 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #32 | C[Si](C)(C)NC1=NC(=O)C2=C(N([Si](C)(C)C)CC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C3)N2)N1[Si](C)(C)C | 4353.0 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #32 | C[Si](C)(C)NC1=NC(=O)C2=C(N([Si](C)(C)C)CC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C3)N2)N1[Si](C)(C)C | 6662.8 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #33 | C[Si](C)(C)NC1=NC(=O)C2=C(N([Si](C)(C)C)CC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C3)N2[Si](C)(C)C)N1[Si](C)(C)C | 4545.4 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #33 | C[Si](C)(C)NC1=NC(=O)C2=C(N([Si](C)(C)C)CC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C3)N2[Si](C)(C)C)N1[Si](C)(C)C | 4314.9 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #33 | C[Si](C)(C)NC1=NC(=O)C2=C(N([Si](C)(C)C)CC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C3)N2[Si](C)(C)C)N1[Si](C)(C)C | 5723.7 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #34 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C3)N2[Si](C)(C)C)N1[Si](C)(C)C | 4532.7 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #34 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C3)N2[Si](C)(C)C)N1[Si](C)(C)C | 4253.5 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #34 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C3)N2[Si](C)(C)C)N1[Si](C)(C)C | 6033.3 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #35 | C[Si](C)(C)NC1=NC(=O)C2=C([NH]1)N([Si](C)(C)C)CC(CN(C=O)C1=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)N2[Si](C)(C)C | 4410.7 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #35 | C[Si](C)(C)NC1=NC(=O)C2=C([NH]1)N([Si](C)(C)C)CC(CN(C=O)C1=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)N2[Si](C)(C)C | 4224.3 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #35 | C[Si](C)(C)NC1=NC(=O)C2=C([NH]1)N([Si](C)(C)C)CC(CN(C=O)C1=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)N2[Si](C)(C)C | 5760.8 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #36 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(C(=O)N=C(N)N3[Si](C)(C)C)N2[Si](C)(C)C)C=C1)[Si](C)(C)C | 4348.6 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #36 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(C(=O)N=C(N)N3[Si](C)(C)C)N2[Si](C)(C)C)C=C1)[Si](C)(C)C | 4212.1 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #36 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C)C3=C(C(=O)N=C(N)N3[Si](C)(C)C)N2[Si](C)(C)C)C=C1)[Si](C)(C)C | 6028.6 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #37 | C[Si](C)(C)N1CC(CN(C=O)C2=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C2)NC2=C1N([Si](C)(C)C)C(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O | 4523.6 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #37 | C[Si](C)(C)N1CC(CN(C=O)C2=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C2)NC2=C1N([Si](C)(C)C)C(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O | 4474.9 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #37 | C[Si](C)(C)N1CC(CN(C=O)C2=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C2)NC2=C1N([Si](C)(C)C)C(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O | 6512.2 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #38 | C[Si](C)(C)N1CC(CN(C=O)C2=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C2)N([Si](C)(C)C)C2=C1N([Si](C)(C)C)C(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O | 4613.5 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #38 | C[Si](C)(C)N1CC(CN(C=O)C2=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C2)N([Si](C)(C)C)C2=C1N([Si](C)(C)C)C(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O | 4472.7 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #38 | C[Si](C)(C)N1CC(CN(C=O)C2=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C2)N([Si](C)(C)C)C2=C1N([Si](C)(C)C)C(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O | 5671.9 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #39 | C[Si](C)(C)N1C2=C(NCC1CN(C=O)C1=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1)N([Si](C)(C)C)C(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O | 4557.2 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #39 | C[Si](C)(C)N1C2=C(NCC1CN(C=O)C1=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1)N([Si](C)(C)C)C(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O | 4411.4 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #39 | C[Si](C)(C)N1C2=C(NCC1CN(C=O)C1=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1)N([Si](C)(C)C)C(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O | 5947.8 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #4 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]3)N2[Si](C)(C)C)C=C1)C(=O)O[Si](C)(C)C | 4531.7 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #4 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]3)N2[Si](C)(C)C)C=C1)C(=O)O[Si](C)(C)C | 4235.1 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #4 | C[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]3)N2[Si](C)(C)C)C=C1)C(=O)O[Si](C)(C)C | 5797.0 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #40 | C[Si](C)(C)N1CC(CN(C=O)C2=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C2)N([Si](C)(C)C)C2=C1[NH]C(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O | 4433.6 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #40 | C[Si](C)(C)N1CC(CN(C=O)C2=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C2)N([Si](C)(C)C)C2=C1[NH]C(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O | 4381.2 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #40 | C[Si](C)(C)N1CC(CN(C=O)C2=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C2)N([Si](C)(C)C)C2=C1[NH]C(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O | 5686.3 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #41 | C[Si](C)(C)NC1=NC(=O)C2=C(N([Si](C)(C)C)CC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C3)N2[Si](C)(C)C)N1[Si](C)(C)C | 4500.0 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #41 | C[Si](C)(C)NC1=NC(=O)C2=C(N([Si](C)(C)C)CC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C3)N2[Si](C)(C)C)N1[Si](C)(C)C | 4351.8 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #41 | C[Si](C)(C)NC1=NC(=O)C2=C(N([Si](C)(C)C)CC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C3)N2[Si](C)(C)C)N1[Si](C)(C)C | 5751.8 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #5 | C[Si](C)(C)NC1=NC(=O)C2=C(N([Si](C)(C)C)CC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C3)N2)N1[Si](C)(C)C | 4511.3 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #5 | C[Si](C)(C)NC1=NC(=O)C2=C(N([Si](C)(C)C)CC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C3)N2)N1[Si](C)(C)C | 4327.1 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #5 | C[Si](C)(C)NC1=NC(=O)C2=C(N([Si](C)(C)C)CC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C3)N2)N1[Si](C)(C)C | 6731.1 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #6 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C3)N2)N1[Si](C)(C)C | 4510.2 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #6 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C3)N2)N1[Si](C)(C)C | 4175.9 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #6 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C3)N2)N1[Si](C)(C)C | 7012.0 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #7 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C3)N2[Si](C)(C)C)N1[Si](C)(C)C | 4561.0 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #7 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C3)N2[Si](C)(C)C)N1[Si](C)(C)C | 4196.9 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #7 | C[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C3)N2[Si](C)(C)C)N1[Si](C)(C)C | 6026.1 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #8 | C[Si](C)(C)NC1=NC(=O)C2=C([NH]1)N([Si](C)(C)C)CC(CN(C=O)C1=CC=C(C(=O)N([C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)N2 | 4396.9 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #8 | C[Si](C)(C)NC1=NC(=O)C2=C([NH]1)N([Si](C)(C)C)CC(CN(C=O)C1=CC=C(C(=O)N([C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)N2 | 4243.7 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #8 | C[Si](C)(C)NC1=NC(=O)C2=C([NH]1)N([Si](C)(C)C)CC(CN(C=O)C1=CC=C(C(=O)N([C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)N2 | 6937.9 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #9 | C[Si](C)(C)NC1=NC(=O)C2=C([NH]1)N([Si](C)(C)C)CC(CN(C=O)C1=CC=C(C(=O)N[C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1)N2[Si](C)(C)C | 4433.3 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #9 | C[Si](C)(C)NC1=NC(=O)C2=C([NH]1)N([Si](C)(C)C)CC(CN(C=O)C1=CC=C(C(=O)N[C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1)N2[Si](C)(C)C | 4184.3 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,5TMS,isomer #9 | C[Si](C)(C)NC1=NC(=O)C2=C([NH]1)N([Si](C)(C)C)CC(CN(C=O)C1=CC=C(C(=O)N[C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1)N2[Si](C)(C)C | 5741.6 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,1TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N)[NH]3)C=C1)C(=O)O | 5241.3 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,1TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)OC(=O)[C@H](CCC(=O)O)NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N)[NH]3)C=C1 | 5209.9 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,1TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C3)N2)[NH]1 | 5282.2 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,1TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)N1CC(CN(C=O)C2=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C2)NC2=C1[NH]C(N)=NC2=O | 5124.0 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,1TBDMS,isomer #5 | CC(C)(C)[Si](C)(C)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N)[NH]3)C=C1)[C@@H](CCC(=O)O)C(=O)O | 5176.8 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,1TBDMS,isomer #6 | CC(C)(C)[Si](C)(C)N1C2=C(NCC1CN(C=O)C1=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C1)[NH]C(N)=NC2=O | 5187.2 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,1TBDMS,isomer #7 | CC(C)(C)[Si](C)(C)N1C(N)=NC(=O)C2=C1NCC(CN(C=O)C1=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C1)N2 | 5120.8 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,2TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N)[NH]3)C=C1)C(=O)O[Si](C)(C)C(C)(C)C | 5299.5 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,2TBDMS,isomer #10 | CC(C)(C)[Si](C)(C)OC(=O)[C@H](CCC(=O)O)NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N)N3[Si](C)(C)C(C)(C)C)C=C1 | 5229.4 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,2TBDMS,isomer #11 | CC(C)(C)[Si](C)(C)OC(=O)[C@H](CCC(=O)O)NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N)[NH]3)N2[Si](C)(C)C(C)(C)C)C=C1 | 5227.8 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,2TBDMS,isomer #12 | CC(C)(C)[Si](C)(C)N(C1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C3)N2)[NH]1)[Si](C)(C)C(C)(C)C | 5364.1 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,2TBDMS,isomer #13 | CC(C)(C)[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C3)N2)N1[Si](C)(C)C(C)(C)C | 5353.3 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,2TBDMS,isomer #14 | CC(C)(C)[Si](C)(C)NC1=NC(=O)C2=C([NH]1)N([Si](C)(C)C(C)(C)C)CC(CN(C=O)C1=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C1)N2 | 5268.9 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,2TBDMS,isomer #15 | CC(C)(C)[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C(C)(C)C)C=C3)N2)[NH]1 | 5310.2 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,2TBDMS,isomer #16 | CC(C)(C)[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C3)N2[Si](C)(C)C(C)(C)C)[NH]1 | 5366.4 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,2TBDMS,isomer #17 | CC(C)(C)[Si](C)(C)N1CC(CN(C=O)C2=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C(C)(C)C)C=C2)NC2=C1[NH]C(N)=NC2=O | 5131.4 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,2TBDMS,isomer #18 | CC(C)(C)[Si](C)(C)N1CC(CN(C=O)C2=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C2)N([Si](C)(C)C(C)(C)C)C2=C1[NH]C(N)=NC2=O | 5170.9 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,2TBDMS,isomer #19 | CC(C)(C)[Si](C)(C)N1CC(CN(C=O)C2=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C2)NC2=C1N([Si](C)(C)C(C)(C)C)C(N)=NC2=O | 5145.9 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,2TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O)C=C3)N2)[NH]1 | 5360.8 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,2TBDMS,isomer #20 | CC(C)(C)[Si](C)(C)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N)N3[Si](C)(C)C(C)(C)C)C=C1)[C@@H](CCC(=O)O)C(=O)O | 5173.3 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,2TBDMS,isomer #21 | CC(C)(C)[Si](C)(C)N1C2=C(NCC1CN(C=O)C1=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C(C)(C)C)C=C1)[NH]C(N)=NC2=O | 5196.3 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,2TBDMS,isomer #22 | CC(C)(C)[Si](C)(C)N1C2=C(NCC1CN(C=O)C1=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C1)N([Si](C)(C)C(C)(C)C)C(N)=NC2=O | 5234.2 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,2TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OC(=O)CC[C@@H](C(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N)[NH]3)C=C1)[Si](C)(C)C(C)(C)C | 5237.6 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,2TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C(C)(C)C)C3=C(N2)C(=O)N=C(N)[NH]3)C=C1)C(=O)O | 5196.1 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,2TBDMS,isomer #5 | CC(C)(C)[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N)N3[Si](C)(C)C(C)(C)C)C=C1)C(=O)O | 5254.4 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,2TBDMS,isomer #6 | CC(C)(C)[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N)[NH]3)N2[Si](C)(C)C(C)(C)C)C=C1)C(=O)O | 5240.4 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,2TBDMS,isomer #7 | CC(C)(C)[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O[Si](C)(C)C(C)(C)C)C=C3)N2)[NH]1 | 5343.7 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,2TBDMS,isomer #8 | CC(C)(C)[Si](C)(C)OC(=O)[C@H](CCC(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N)[NH]3)C=C1)[Si](C)(C)C(C)(C)C | 5240.7 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,2TBDMS,isomer #9 | CC(C)(C)[Si](C)(C)OC(=O)[C@H](CCC(=O)O)NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C(C)(C)C)C3=C(N2)C(=O)N=C(N)[NH]3)C=C1 | 5175.0 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O[Si](C)(C)C(C)(C)C)C=C3)N2)[NH]1 | 5357.2 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O[Si](C)(C)C(C)(C)C)C=C3)N2)[NH]1 | 4797.3 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O[Si](C)(C)C(C)(C)C)C=C3)N2)[NH]1 | 7668.9 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #10 | CC(C)(C)[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O)C=C3)N2[Si](C)(C)C(C)(C)C)[NH]1 | 5371.6 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #10 | CC(C)(C)[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O)C=C3)N2[Si](C)(C)C(C)(C)C)[NH]1 | 4880.3 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #10 | CC(C)(C)[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O)C=C3)N2[Si](C)(C)C(C)(C)C)[NH]1 | 6547.0 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #11 | CC(C)(C)[Si](C)(C)OC(=O)CC[C@@H](C(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C(C)(C)C)C3=C(N2)C(=O)N=C(N)[NH]3)C=C1)[Si](C)(C)C(C)(C)C | 5160.4 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #11 | CC(C)(C)[Si](C)(C)OC(=O)CC[C@@H](C(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C(C)(C)C)C3=C(N2)C(=O)N=C(N)[NH]3)C=C1)[Si](C)(C)C(C)(C)C | 4696.3 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #11 | CC(C)(C)[Si](C)(C)OC(=O)CC[C@@H](C(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C(C)(C)C)C3=C(N2)C(=O)N=C(N)[NH]3)C=C1)[Si](C)(C)C(C)(C)C | 6711.9 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #12 | CC(C)(C)[Si](C)(C)OC(=O)CC[C@@H](C(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N)N3[Si](C)(C)C(C)(C)C)C=C1)[Si](C)(C)C(C)(C)C | 5232.2 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #12 | CC(C)(C)[Si](C)(C)OC(=O)CC[C@@H](C(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N)N3[Si](C)(C)C(C)(C)C)C=C1)[Si](C)(C)C(C)(C)C | 4636.9 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #12 | CC(C)(C)[Si](C)(C)OC(=O)CC[C@@H](C(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N)N3[Si](C)(C)C(C)(C)C)C=C1)[Si](C)(C)C(C)(C)C | 6823.1 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #13 | CC(C)(C)[Si](C)(C)OC(=O)CC[C@@H](C(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N)[NH]3)N2[Si](C)(C)C(C)(C)C)C=C1)[Si](C)(C)C(C)(C)C | 5196.0 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #13 | CC(C)(C)[Si](C)(C)OC(=O)CC[C@@H](C(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N)[NH]3)N2[Si](C)(C)C(C)(C)C)C=C1)[Si](C)(C)C(C)(C)C | 4684.1 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #13 | CC(C)(C)[Si](C)(C)OC(=O)CC[C@@H](C(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N)[NH]3)N2[Si](C)(C)C(C)(C)C)C=C1)[Si](C)(C)C(C)(C)C | 6385.3 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #14 | CC(C)(C)[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C(C)(C)C)C3=C(N2)C(=O)N=C(N)N3[Si](C)(C)C(C)(C)C)C=C1)C(=O)O | 5245.6 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #14 | CC(C)(C)[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C(C)(C)C)C3=C(N2)C(=O)N=C(N)N3[Si](C)(C)C(C)(C)C)C=C1)C(=O)O | 4801.9 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #14 | CC(C)(C)[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C(C)(C)C)C3=C(N2)C(=O)N=C(N)N3[Si](C)(C)C(C)(C)C)C=C1)C(=O)O | 6792.4 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #15 | CC(C)(C)[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C(C)(C)C)C3=C(C(=O)N=C(N)[NH]3)N2[Si](C)(C)C(C)(C)C)C=C1)C(=O)O | 5198.6 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #15 | CC(C)(C)[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C(C)(C)C)C3=C(C(=O)N=C(N)[NH]3)N2[Si](C)(C)C(C)(C)C)C=C1)C(=O)O | 4762.0 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #15 | CC(C)(C)[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C(C)(C)C)C3=C(C(=O)N=C(N)[NH]3)N2[Si](C)(C)C(C)(C)C)C=C1)C(=O)O | 6388.4 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #16 | CC(C)(C)[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N)N3[Si](C)(C)C(C)(C)C)N2[Si](C)(C)C(C)(C)C)C=C1)C(=O)O | 5316.7 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #16 | CC(C)(C)[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N)N3[Si](C)(C)C(C)(C)C)N2[Si](C)(C)C(C)(C)C)C=C1)C(=O)O | 4769.4 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #16 | CC(C)(C)[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N)N3[Si](C)(C)C(C)(C)C)N2[Si](C)(C)C(C)(C)C)C=C1)C(=O)O | 6541.2 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #17 | CC(C)(C)[Si](C)(C)OC(=O)[C@H](CCC(=O)O)NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[NH]3)C=C1 | 5376.3 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #17 | CC(C)(C)[Si](C)(C)OC(=O)[C@H](CCC(=O)O)NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[NH]3)C=C1 | 4848.4 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #17 | CC(C)(C)[Si](C)(C)OC(=O)[C@H](CCC(=O)O)NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[NH]3)C=C1 | 7369.0 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #18 | CC(C)(C)[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O[Si](C)(C)C(C)(C)C)C=C3)N2)N1[Si](C)(C)C(C)(C)C | 5378.3 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #18 | CC(C)(C)[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O[Si](C)(C)C(C)(C)C)C=C3)N2)N1[Si](C)(C)C(C)(C)C | 4794.9 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #18 | CC(C)(C)[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O[Si](C)(C)C(C)(C)C)C=C3)N2)N1[Si](C)(C)C(C)(C)C | 7305.1 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #19 | CC(C)(C)[Si](C)(C)NC1=NC(=O)C2=C([NH]1)N([Si](C)(C)C(C)(C)C)CC(CN(C=O)C1=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O[Si](C)(C)C(C)(C)C)C=C1)N2 | 5284.1 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #19 | CC(C)(C)[Si](C)(C)NC1=NC(=O)C2=C([NH]1)N([Si](C)(C)C(C)(C)C)CC(CN(C=O)C1=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O[Si](C)(C)C(C)(C)C)C=C1)N2 | 4875.6 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #19 | CC(C)(C)[Si](C)(C)NC1=NC(=O)C2=C([NH]1)N([Si](C)(C)C(C)(C)C)CC(CN(C=O)C1=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O[Si](C)(C)C(C)(C)C)C=C1)N2 | 7351.7 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)OC(=O)CC[C@@H](C(=O)O[Si](C)(C)C(C)(C)C)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N)[NH]3)C=C1)[Si](C)(C)C(C)(C)C | 5270.4 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)OC(=O)CC[C@@H](C(=O)O[Si](C)(C)C(C)(C)C)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N)[NH]3)C=C1)[Si](C)(C)C(C)(C)C | 4597.2 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)OC(=O)CC[C@@H](C(=O)O[Si](C)(C)C(C)(C)C)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N)[NH]3)C=C1)[Si](C)(C)C(C)(C)C | 6783.7 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #20 | CC(C)(C)[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)C=C3)N2)[NH]1 | 5295.9 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #20 | CC(C)(C)[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)C=C3)N2)[NH]1 | 4765.1 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #20 | CC(C)(C)[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)C=C3)N2)[NH]1 | 7587.4 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #21 | CC(C)(C)[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O[Si](C)(C)C(C)(C)C)C=C3)N2[Si](C)(C)C(C)(C)C)[NH]1 | 5365.5 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #21 | CC(C)(C)[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O[Si](C)(C)C(C)(C)C)C=C3)N2[Si](C)(C)C(C)(C)C)[NH]1 | 4864.9 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #21 | CC(C)(C)[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O[Si](C)(C)C(C)(C)C)C=C3)N2[Si](C)(C)C(C)(C)C)[NH]1 | 6506.4 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #22 | CC(C)(C)[Si](C)(C)OC(=O)[C@H](CCC(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C(C)(C)C)C3=C(N2)C(=O)N=C(N)[NH]3)C=C1)[Si](C)(C)C(C)(C)C | 5154.8 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #22 | CC(C)(C)[Si](C)(C)OC(=O)[C@H](CCC(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C(C)(C)C)C3=C(N2)C(=O)N=C(N)[NH]3)C=C1)[Si](C)(C)C(C)(C)C | 4681.5 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #22 | CC(C)(C)[Si](C)(C)OC(=O)[C@H](CCC(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C(C)(C)C)C3=C(N2)C(=O)N=C(N)[NH]3)C=C1)[Si](C)(C)C(C)(C)C | 6690.4 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #23 | CC(C)(C)[Si](C)(C)OC(=O)[C@H](CCC(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N)N3[Si](C)(C)C(C)(C)C)C=C1)[Si](C)(C)C(C)(C)C | 5229.2 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #23 | CC(C)(C)[Si](C)(C)OC(=O)[C@H](CCC(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N)N3[Si](C)(C)C(C)(C)C)C=C1)[Si](C)(C)C(C)(C)C | 4621.7 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #23 | CC(C)(C)[Si](C)(C)OC(=O)[C@H](CCC(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N)N3[Si](C)(C)C(C)(C)C)C=C1)[Si](C)(C)C(C)(C)C | 6802.0 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #24 | CC(C)(C)[Si](C)(C)OC(=O)[C@H](CCC(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N)[NH]3)N2[Si](C)(C)C(C)(C)C)C=C1)[Si](C)(C)C(C)(C)C | 5197.9 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #24 | CC(C)(C)[Si](C)(C)OC(=O)[C@H](CCC(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N)[NH]3)N2[Si](C)(C)C(C)(C)C)C=C1)[Si](C)(C)C(C)(C)C | 4677.4 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #24 | CC(C)(C)[Si](C)(C)OC(=O)[C@H](CCC(=O)O)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N)[NH]3)N2[Si](C)(C)C(C)(C)C)C=C1)[Si](C)(C)C(C)(C)C | 6363.8 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #25 | CC(C)(C)[Si](C)(C)OC(=O)[C@H](CCC(=O)O)NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C(C)(C)C)C3=C(N2)C(=O)N=C(N)N3[Si](C)(C)C(C)(C)C)C=C1 | 5224.9 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #25 | CC(C)(C)[Si](C)(C)OC(=O)[C@H](CCC(=O)O)NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C(C)(C)C)C3=C(N2)C(=O)N=C(N)N3[Si](C)(C)C(C)(C)C)C=C1 | 4777.5 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #25 | CC(C)(C)[Si](C)(C)OC(=O)[C@H](CCC(=O)O)NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C(C)(C)C)C3=C(N2)C(=O)N=C(N)N3[Si](C)(C)C(C)(C)C)C=C1 | 6746.1 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #26 | CC(C)(C)[Si](C)(C)OC(=O)[C@H](CCC(=O)O)NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C(C)(C)C)C3=C(C(=O)N=C(N)[NH]3)N2[Si](C)(C)C(C)(C)C)C=C1 | 5180.5 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #26 | CC(C)(C)[Si](C)(C)OC(=O)[C@H](CCC(=O)O)NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C(C)(C)C)C3=C(C(=O)N=C(N)[NH]3)N2[Si](C)(C)C(C)(C)C)C=C1 | 4732.8 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #26 | CC(C)(C)[Si](C)(C)OC(=O)[C@H](CCC(=O)O)NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C(C)(C)C)C3=C(C(=O)N=C(N)[NH]3)N2[Si](C)(C)C(C)(C)C)C=C1 | 6343.3 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #27 | CC(C)(C)[Si](C)(C)OC(=O)[C@H](CCC(=O)O)NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N)N3[Si](C)(C)C(C)(C)C)N2[Si](C)(C)C(C)(C)C)C=C1 | 5295.4 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #27 | CC(C)(C)[Si](C)(C)OC(=O)[C@H](CCC(=O)O)NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N)N3[Si](C)(C)C(C)(C)C)N2[Si](C)(C)C(C)(C)C)C=C1 | 4751.8 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #27 | CC(C)(C)[Si](C)(C)OC(=O)[C@H](CCC(=O)O)NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N)N3[Si](C)(C)C(C)(C)C)N2[Si](C)(C)C(C)(C)C)C=C1 | 6494.6 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #28 | CC(C)(C)[Si](C)(C)N(C1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C3)N2)N1[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 5430.5 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #28 | CC(C)(C)[Si](C)(C)N(C1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C3)N2)N1[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 4867.1 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #28 | CC(C)(C)[Si](C)(C)N(C1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C3)N2)N1[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 7226.8 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #29 | CC(C)(C)[Si](C)(C)N1CC(CN(C=O)C2=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C2)NC2=C1[NH]C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)=NC2=O | 5341.5 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #29 | CC(C)(C)[Si](C)(C)N1CC(CN(C=O)C2=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C2)NC2=C1[NH]C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)=NC2=O | 4919.4 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #29 | CC(C)(C)[Si](C)(C)N1CC(CN(C=O)C2=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C2)NC2=C1[NH]C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)=NC2=O | 7234.7 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C(C)(C)C)C3=C(N2)C(=O)N=C(N)[NH]3)C=C1)C(=O)O[Si](C)(C)C(C)(C)C | 5201.7 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C(C)(C)C)C3=C(N2)C(=O)N=C(N)[NH]3)C=C1)C(=O)O[Si](C)(C)C(C)(C)C | 4702.8 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CN([Si](C)(C)C(C)(C)C)C3=C(N2)C(=O)N=C(N)[NH]3)C=C1)C(=O)O[Si](C)(C)C(C)(C)C | 6747.7 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #30 | CC(C)(C)[Si](C)(C)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[NH]3)C=C1)[C@@H](CCC(=O)O)C(=O)O | 5328.4 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #30 | CC(C)(C)[Si](C)(C)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[NH]3)C=C1)[C@@H](CCC(=O)O)C(=O)O | 4832.0 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #30 | CC(C)(C)[Si](C)(C)N(C(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[NH]3)C=C1)[C@@H](CCC(=O)O)C(=O)O | 7320.8 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #31 | CC(C)(C)[Si](C)(C)N1C2=C(NCC1CN(C=O)C1=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C1)[NH]C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)=NC2=O | 5427.7 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #31 | CC(C)(C)[Si](C)(C)N1C2=C(NCC1CN(C=O)C1=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C1)[NH]C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)=NC2=O | 4974.7 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #31 | CC(C)(C)[Si](C)(C)N1C2=C(NCC1CN(C=O)C1=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C1)[NH]C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)=NC2=O | 6403.8 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #32 | CC(C)(C)[Si](C)(C)NC1=NC(=O)C2=C(N([Si](C)(C)C(C)(C)C)CC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C3)N2)N1[Si](C)(C)C(C)(C)C | 5356.3 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #32 | CC(C)(C)[Si](C)(C)NC1=NC(=O)C2=C(N([Si](C)(C)C(C)(C)C)CC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C3)N2)N1[Si](C)(C)C(C)(C)C | 4961.7 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #32 | CC(C)(C)[Si](C)(C)NC1=NC(=O)C2=C(N([Si](C)(C)C(C)(C)C)CC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C3)N2)N1[Si](C)(C)C(C)(C)C | 7089.3 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #33 | CC(C)(C)[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C(C)(C)C)C=C3)N2)N1[Si](C)(C)C(C)(C)C | 5334.2 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #33 | CC(C)(C)[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C(C)(C)C)C=C3)N2)N1[Si](C)(C)C(C)(C)C | 4784.2 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #33 | CC(C)(C)[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C(C)(C)C)C=C3)N2)N1[Si](C)(C)C(C)(C)C | 7257.0 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #34 | CC(C)(C)[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C3)N2[Si](C)(C)C(C)(C)C)N1[Si](C)(C)C(C)(C)C | 5454.6 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #34 | CC(C)(C)[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C3)N2[Si](C)(C)C(C)(C)C)N1[Si](C)(C)C(C)(C)C | 4945.3 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #34 | CC(C)(C)[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C3)N2[Si](C)(C)C(C)(C)C)N1[Si](C)(C)C(C)(C)C | 6469.1 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #35 | CC(C)(C)[Si](C)(C)NC1=NC(=O)C2=C([NH]1)N([Si](C)(C)C(C)(C)C)CC(CN(C=O)C1=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C(C)(C)C)C=C1)N2 | 5260.7 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #35 | CC(C)(C)[Si](C)(C)NC1=NC(=O)C2=C([NH]1)N([Si](C)(C)C(C)(C)C)CC(CN(C=O)C1=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C(C)(C)C)C=C1)N2 | 4870.8 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #35 | CC(C)(C)[Si](C)(C)NC1=NC(=O)C2=C([NH]1)N([Si](C)(C)C(C)(C)C)CC(CN(C=O)C1=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C(C)(C)C)C=C1)N2 | 7307.0 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #36 | CC(C)(C)[Si](C)(C)NC1=NC(=O)C2=C([NH]1)N([Si](C)(C)C(C)(C)C)CC(CN(C=O)C1=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C1)N2[Si](C)(C)C(C)(C)C | 5361.3 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #36 | CC(C)(C)[Si](C)(C)NC1=NC(=O)C2=C([NH]1)N([Si](C)(C)C(C)(C)C)CC(CN(C=O)C1=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C1)N2[Si](C)(C)C(C)(C)C | 4926.1 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #36 | CC(C)(C)[Si](C)(C)NC1=NC(=O)C2=C([NH]1)N([Si](C)(C)C(C)(C)C)CC(CN(C=O)C1=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C1)N2[Si](C)(C)C(C)(C)C | 6358.2 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #37 | CC(C)(C)[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C(C)(C)C)C=C3)N2[Si](C)(C)C(C)(C)C)[NH]1 | 5352.6 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #37 | CC(C)(C)[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C(C)(C)C)C=C3)N2[Si](C)(C)C(C)(C)C)[NH]1 | 4866.0 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #37 | CC(C)(C)[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C(C)(C)C)C=C3)N2[Si](C)(C)C(C)(C)C)[NH]1 | 6512.1 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #38 | CC(C)(C)[Si](C)(C)N1CC(CN(C=O)C2=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C(C)(C)C)C=C2)N([Si](C)(C)C(C)(C)C)C2=C1[NH]C(N)=NC2=O | 5158.7 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #38 | CC(C)(C)[Si](C)(C)N1CC(CN(C=O)C2=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C(C)(C)C)C=C2)N([Si](C)(C)C(C)(C)C)C2=C1[NH]C(N)=NC2=O | 4730.7 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #38 | CC(C)(C)[Si](C)(C)N1CC(CN(C=O)C2=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C(C)(C)C)C=C2)N([Si](C)(C)C(C)(C)C)C2=C1[NH]C(N)=NC2=O | 6333.4 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #39 | CC(C)(C)[Si](C)(C)N1CC(CN(C=O)C2=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C(C)(C)C)C=C2)NC2=C1N([Si](C)(C)C(C)(C)C)C(N)=NC2=O | 5191.7 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #39 | CC(C)(C)[Si](C)(C)N1CC(CN(C=O)C2=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C(C)(C)C)C=C2)NC2=C1N([Si](C)(C)C(C)(C)C)C(N)=NC2=O | 4767.3 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #39 | CC(C)(C)[Si](C)(C)N1CC(CN(C=O)C2=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C(C)(C)C)C=C2)NC2=C1N([Si](C)(C)C(C)(C)C)C(N)=NC2=O | 6724.1 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N)N3[Si](C)(C)C(C)(C)C)C=C1)C(=O)O[Si](C)(C)C(C)(C)C | 5271.4 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N)N3[Si](C)(C)C(C)(C)C)C=C1)C(=O)O[Si](C)(C)C(C)(C)C | 4648.4 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N)N3[Si](C)(C)C(C)(C)C)C=C1)C(=O)O[Si](C)(C)C(C)(C)C | 6854.0 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #40 | CC(C)(C)[Si](C)(C)N1CC(CN(C=O)C2=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C2)N([Si](C)(C)C(C)(C)C)C2=C1N([Si](C)(C)C(C)(C)C)C(N)=NC2=O | 5288.3 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #40 | CC(C)(C)[Si](C)(C)N1CC(CN(C=O)C2=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C2)N([Si](C)(C)C(C)(C)C)C2=C1N([Si](C)(C)C(C)(C)C)C(N)=NC2=O | 4857.0 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #40 | CC(C)(C)[Si](C)(C)N1CC(CN(C=O)C2=CC=C(C(=O)N[C@@H](CCC(=O)O)C(=O)O)C=C2)N([Si](C)(C)C(C)(C)C)C2=C1N([Si](C)(C)C(C)(C)C)C(N)=NC2=O | 6453.1 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #41 | CC(C)(C)[Si](C)(C)N1C2=C(NCC1CN(C=O)C1=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C(C)(C)C)C=C1)N([Si](C)(C)C(C)(C)C)C(N)=NC2=O | 5254.6 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #41 | CC(C)(C)[Si](C)(C)N1C2=C(NCC1CN(C=O)C1=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C(C)(C)C)C=C1)N([Si](C)(C)C(C)(C)C)C(N)=NC2=O | 4770.7 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #41 | CC(C)(C)[Si](C)(C)N1C2=C(NCC1CN(C=O)C1=CC=C(C(=O)N([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C(C)(C)C)C=C1)N([Si](C)(C)C(C)(C)C)C(N)=NC2=O | 6517.4 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #5 | CC(C)(C)[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N)[NH]3)N2[Si](C)(C)C(C)(C)C)C=C1)C(=O)O[Si](C)(C)C(C)(C)C | 5244.6 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #5 | CC(C)(C)[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N)[NH]3)N2[Si](C)(C)C(C)(C)C)C=C1)C(=O)O[Si](C)(C)C(C)(C)C | 4690.3 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #5 | CC(C)(C)[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(C(=O)N=C(N)[NH]3)N2[Si](C)(C)C(C)(C)C)C=C1)C(=O)O[Si](C)(C)C(C)(C)C | 6404.1 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #6 | CC(C)(C)[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[NH]3)C=C1)C(=O)O | 5392.1 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #6 | CC(C)(C)[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[NH]3)C=C1)C(=O)O | 4877.1 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #6 | CC(C)(C)[Si](C)(C)OC(=O)CC[C@H](NC(=O)C1=CC=C(N(C=O)CC2CNC3=C(N2)C(=O)N=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[NH]3)C=C1)C(=O)O | 7412.5 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #7 | CC(C)(C)[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O)C=C3)N2)N1[Si](C)(C)C(C)(C)C | 5394.8 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #7 | CC(C)(C)[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O)C=C3)N2)N1[Si](C)(C)C(C)(C)C | 4817.6 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #7 | CC(C)(C)[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N[C@@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O)C=C3)N2)N1[Si](C)(C)C(C)(C)C | 7346.4 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #8 | CC(C)(C)[Si](C)(C)NC1=NC(=O)C2=C([NH]1)N([Si](C)(C)C(C)(C)C)CC(CN(C=O)C1=CC=C(C(=O)N[C@@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O)C=C1)N2 | 5311.5 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #8 | CC(C)(C)[Si](C)(C)NC1=NC(=O)C2=C([NH]1)N([Si](C)(C)C(C)(C)C)CC(CN(C=O)C1=CC=C(C(=O)N[C@@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O)C=C1)N2 | 4900.2 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #8 | CC(C)(C)[Si](C)(C)NC1=NC(=O)C2=C([NH]1)N([Si](C)(C)C(C)(C)C)CC(CN(C=O)C1=CC=C(C(=O)N[C@@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O)C=C1)N2 | 7392.9 | Standard polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #9 | CC(C)(C)[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O)[Si](C)(C)C(C)(C)C)C=C3)N2)[NH]1 | 5289.6 | Semi standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #9 | CC(C)(C)[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O)[Si](C)(C)C(C)(C)C)C=C3)N2)[NH]1 | 4778.7 | Standard non polar | 33892256 |
| 10-Formyltetrahydrofolate,3TBDMS,isomer #9 | CC(C)(C)[Si](C)(C)NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C(=O)N([C@@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O)[Si](C)(C)C(C)(C)C)C=C3)N2)[NH]1 | 7608.1 | Standard polar | 33892256 |