| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Detected and Quantified |
|---|
| Creation Date | 2006-05-22 14:17:32 UTC |
|---|
| Update Date | 2023-02-21 17:16:04 UTC |
|---|
| HMDB ID | HMDB0002024 |
|---|
| Secondary Accession Numbers | - HMDB0060261
- HMDB02024
- HMDB60261
|
|---|
| Metabolite Identification |
|---|
| Common Name | Imidazoleacetic acid |
|---|
| Description | Imidazol-4-ylacetic acid is a monocarboxylic acid that is acetic acid in which one of the methyl hydrogens has been replaced by an imidazol-4-yl group. It has a role as a mouse metabolite. It is a monocarboxylic acid and a member of imidazoles. It derives from an acetic acid. It is a conjugate acid of an imidazol-4-ylacetate. It is a tautomer of an imidazol-5-ylacetic acid and a 2H-imidazol-4-ylacetic acid. Imidazoleacetic acid, also known as 4(5)-imidazoleacetate or IAA, belongs to the class of organic compounds known as imidazolyl carboxylic acids and derivatives. These are organic compounds containing a carboxylic acid chain (of at least 2 carbon atoms) linked to an imidazole ring. Imidazoleacetic acid exists in all living organisms, ranging from bacteria to humans. imidazoleacetic acid can be biosynthesized from imidazole-4-acetaldehyde through its interaction with the enzyme aldehyde dehydrogenase, mitochondrial. In humans, imidazoleacetic acid is involved in histidine metabolism. Outside of the human body, Imidazoleacetic acid has been detected, but not quantified in several different foods, such as chinese cinnamons, jostaberries, vanilla, butternut squash, and red rices. Imidazoleacetic acid is a potentially toxic compound. Imidazoleacetic acid is a metabolite product of Histamine metabolism. |
|---|
| Structure | InChI=1S/C5H6N2O2/c8-5(9)1-4-2-6-3-7-4/h2-3H,1H2,(H,6,7)(H,8,9) |
|---|
| Synonyms | | Value | Source |
|---|
| 1H-Imidazole-4-acetic acid | ChEBI | | 4(5)-Imidazoleacetate | ChEBI | | 4-Imidazoleacetate | ChEBI | | Imidazole-4-acetate | ChEBI | | 1H-Imidazole-4-acetate | Generator | | 4(5)-Imidazoleacetic acid | Generator | | 4-Imidazoleacetic acid | Generator | | Imidazole-4-acetic acid | Generator | | Imidazoleacetate | Generator | | 1H-Imidazol-4-ylacetic acid | HMDB | | IAA | HMDB | | IMAC | HMDB | | Imidazol-4-ylacetate | HMDB | | Imidazol-4-ylacetic acid | HMDB | | Imidazole acetate | HMDB | | Imidazolyl-4-acetic acid | HMDB | | IZC | HMDB | | Lopac-I-0375 | HMDB | | Imidazole-4-acetic acid, sodium salt | HMDB | | Imidazole-4-acetic acid hydrochloride | HMDB |
|
|---|
| Chemical Formula | C5H6N2O2 |
|---|
| Average Molecular Weight | 126.1133 |
|---|
| Monoisotopic Molecular Weight | 126.042927446 |
|---|
| IUPAC Name | 2-(1H-imidazol-5-yl)acetic acid |
|---|
| Traditional Name | IMAC |
|---|
| CAS Registry Number | 645-65-8 |
|---|
| SMILES | OC(=O)CC1=CN=CN1 |
|---|
| InChI Identifier | InChI=1S/C5H6N2O2/c8-5(9)1-4-2-6-3-7-4/h2-3H,1H2,(H,6,7)(H,8,9) |
|---|
| InChI Key | PRJKNHOMHKJCEJ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as imidazolyl carboxylic acids and derivatives. These are organic compounds containing a carboxylic acid chain (of at least 2 carbon atoms) linked to an imidazole ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Azoles |
|---|
| Sub Class | Imidazoles |
|---|
| Direct Parent | Imidazolyl carboxylic acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Imidazolyl carboxylic acid derivative
- Heteroaromatic compound
- Azacycle
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Not Available | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Experimental Collision Cross Sections |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Retention Times Underivatized| Chromatographic Method | Retention Time | Reference |
|---|
| Measured using a Waters Acquity ultraperformance liquid chromatography (UPLC) ethylene-bridged hybrid (BEH) C18 column (100 mm × 2.1 mm; 1.7 μmparticle diameter). Predicted by Afia on May 17, 2022. Predicted by Afia on May 17, 2022. | 1.75 minutes | 32390414 | | Predicted by Siyang on May 30, 2022 | 8.6663 minutes | 33406817 | | Predicted by Siyang using ReTip algorithm on June 8, 2022 | 4.74 minutes | 32390414 | | AjsUoB = Accucore 150 Amide HILIC with 10mM Ammonium Formate, 0.1% Formic Acid | 361.3 seconds | 40023050 |
Predicted Kovats Retention IndicesUnderivatizedDerivatized| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| Imidazoleacetic acid,1TMS,isomer #1 | C[Si](C)(C)OC(=O)CC1=CN=C[NH]1 | 1382.6 | Semi standard non polar | 33892256 | | Imidazoleacetic acid,1TMS,isomer #2 | C[Si](C)(C)N1C=NC=C1CC(=O)O | 1577.2 | Semi standard non polar | 33892256 | | Imidazoleacetic acid,2TMS,isomer #1 | C[Si](C)(C)OC(=O)CC1=CN=CN1[Si](C)(C)C | 1588.5 | Semi standard non polar | 33892256 | | Imidazoleacetic acid,2TMS,isomer #1 | C[Si](C)(C)OC(=O)CC1=CN=CN1[Si](C)(C)C | 1540.6 | Standard non polar | 33892256 | | Imidazoleacetic acid,2TMS,isomer #1 | C[Si](C)(C)OC(=O)CC1=CN=CN1[Si](C)(C)C | 1861.2 | Standard polar | 33892256 | | Imidazoleacetic acid,1TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)CC1=CN=C[NH]1 | 1613.4 | Semi standard non polar | 33892256 | | Imidazoleacetic acid,1TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)N1C=NC=C1CC(=O)O | 1856.0 | Semi standard non polar | 33892256 | | Imidazoleacetic acid,2TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)CC1=CN=CN1[Si](C)(C)C(C)(C)C | 2066.7 | Semi standard non polar | 33892256 | | Imidazoleacetic acid,2TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)CC1=CN=CN1[Si](C)(C)C(C)(C)C | 1995.2 | Standard non polar | 33892256 | | Imidazoleacetic acid,2TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)CC1=CN=CN1[Si](C)(C)C(C)(C)C | 2036.6 | Standard polar | 33892256 |
|
|---|
| Spectra |
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental GC-MS | GC-MS Spectrum - Imidazoleacetic acid GC-MS (1 TMS) | splash10-0f89-5900000000-420b0de40d13d8210bbc | 2014-06-16 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Imidazoleacetic acid GC-MS (2 TMS) | splash10-0fc0-5970000000-86a11eed673e756aa38a | 2014-06-16 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Imidazoleacetic acid GC-MS (Non-derivatized) | splash10-0f89-5900000000-420b0de40d13d8210bbc | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Imidazoleacetic acid GC-MS (Non-derivatized) | splash10-0fc0-5970000000-86a11eed673e756aa38a | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Imidazoleacetic acid GC-MS (Non-derivatized) - 70eV, Positive | splash10-001i-9200000000-3560ea9910e3fb51b173 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Imidazoleacetic acid GC-MS (1 TMS) - 70eV, Positive | splash10-00di-8900000000-05046aa1145002d7c5e8 | 2017-10-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Imidazoleacetic acid GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental LC-MS/MS | LC-MS/MS Spectrum - Imidazoleacetic acid Quattro_QQQ 10V, Positive-QTOF (Annotated) | splash10-003r-9600000000-12712603bcc1f0bea126 | 2012-07-25 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Imidazoleacetic acid Quattro_QQQ 25V, Positive-QTOF (Annotated) | splash10-001i-9000000000-fc527ff9b17df8a45f5d | 2012-07-25 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Imidazoleacetic acid Quattro_QQQ 40V, Positive-QTOF (Annotated) | splash10-001i-9000000000-f50438c3b380c53358cb | 2012-07-25 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Imidazoleacetic acid LC-ESI-QQ (API3000, Applied Biosystems) 10V, Positive-QTOF | splash10-004i-0900000000-3e8a5824e3117a9ff630 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Imidazoleacetic acid LC-ESI-QQ (API3000, Applied Biosystems) 20V, Positive-QTOF | splash10-001i-9100000000-bd0eae5cae4e35af3bc9 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Imidazoleacetic acid LC-ESI-QQ (API3000, Applied Biosystems) 30V, Positive-QTOF | splash10-001i-9000000000-029fc71514700cdb7209 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Imidazoleacetic acid LC-ESI-QQ (API3000, Applied Biosystems) 40V, Positive-QTOF | splash10-001i-9000000000-55f8440d265a11022fb9 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Imidazoleacetic acid LC-ESI-QQ (API3000, Applied Biosystems) 50V, Positive-QTOF | splash10-001i-9000000000-2f783a29f0103b0f7b59 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Imidazoleacetic acid , negative-QTOF | splash10-001i-9000000000-536179ad66be87ef6052 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Imidazoleacetic acid LC-ESI-QQ , positive-QTOF | splash10-004i-0900000000-3e8a5824e3117a9ff630 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Imidazoleacetic acid LC-ESI-QQ , positive-QTOF | splash10-001i-9100000000-bd0eae5cae4e35af3bc9 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Imidazoleacetic acid LC-ESI-QQ , positive-QTOF | splash10-001i-9000000000-029fc71514700cdb7209 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Imidazoleacetic acid LC-ESI-QQ , positive-QTOF | splash10-001i-9000000000-55f8440d265a11022fb9 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Imidazoleacetic acid LC-ESI-QQ , positive-QTOF | splash10-001i-9000000000-2f783a29f0103b0f7b59 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Imidazoleacetic acid 0V, Positive-QTOF | splash10-004i-1900000000-2df669861c33e046ef52 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Imidazoleacetic acid 40V, Positive-QTOF | splash10-0f89-9000000000-9ab02097fca907a3b75f | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Imidazoleacetic acid 35V, Negative-QTOF | splash10-001i-9000000000-8c245e2f0f54c3db08d7 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Imidazoleacetic acid 10V, Positive-QTOF | splash10-001i-9100000000-5976268b58ff0c9c054a | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Imidazoleacetic acid 10V, Positive-QTOF | splash10-001i-9100000000-ffb1202ccd976a3c4ac6 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Imidazoleacetic acid 10V, Positive-QTOF | splash10-0a4i-1900000000-4b77cf695394035f5cb9 | 2015-05-26 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Imidazoleacetic acid 20V, Positive-QTOF | splash10-0a4i-8900000000-3a84b22e5a91324c68a8 | 2015-05-26 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Imidazoleacetic acid 40V, Positive-QTOF | splash10-0gc3-9000000000-c43447728a419e4b671a | 2015-05-26 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Imidazoleacetic acid 10V, Negative-QTOF | splash10-004i-3900000000-d826c494ed679b7cd08e | 2015-05-27 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Imidazoleacetic acid 20V, Negative-QTOF | splash10-0560-9800000000-1d0c114437988870749d | 2015-05-27 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Imidazoleacetic acid 40V, Negative-QTOF | splash10-0kal-9100000000-802d3d5a745c25a2b664 | 2015-05-27 | Wishart Lab | View Spectrum |
NMR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Experimental 1D NMR | 1H NMR Spectrum (1D, 500 MHz, H2O, experimental) | 2012-12-05 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Experimental 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, H2O, experimental) | 2012-12-05 | Wishart Lab | View Spectrum |
IR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M-H]-) | 2023-02-03 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M+H]+) | 2023-02-03 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M+Na]+) | 2023-02-03 | FELIX lab | View Spectrum |
|
|---|
| Biological Properties |
|---|
| Cellular Locations | |
|---|
| Biospecimen Locations | - Blood
- Cerebrospinal Fluid (CSF)
- Feces
- Urine
|
|---|
| Tissue Locations | |
|---|
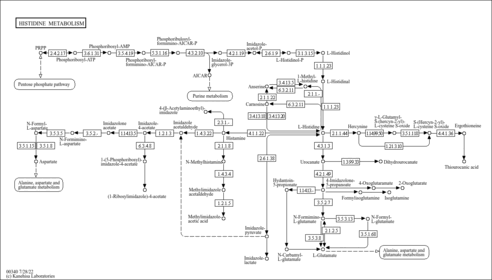
| Pathways | |
|---|
| Normal Concentrations |
|---|
| |
| Blood | Detected and Quantified | 0.10 +/- 0.005 uM | Adult (>18 years old) | Not Specified | Normal | | details | | Blood | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 0.0027 +/- 0.0002 uM | Adult (>18 years old) | Not Specified | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Not Specified | Both | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Urine | Detected and Quantified | 2.602 (0.645-4.525) umol/mmol creatinine | Adult (>18 years old) | Both | Normal | | details | | Urine | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details |
|
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| Associated Disorders and Diseases |
|---|
| Disease References | None |
|---|
| Associated OMIM IDs | None |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| Phenol Explorer Compound ID | Not Available |
|---|
| FooDB ID | FDB030508 |
|---|
| KNApSAcK ID | Not Available |
|---|
| Chemspider ID | 86856 |
|---|
| KEGG Compound ID | C02835 |
|---|
| BioCyc ID | 4-IMIDAZOLEACETATE |
|---|
| BiGG ID | 40666 |
|---|
| Wikipedia Link | Not Available |
|---|
| METLIN ID | 6445 |
|---|
| PubChem Compound | 96215 |
|---|
| PDB ID | Not Available |
|---|
| ChEBI ID | 16974 |
|---|
| Food Biomarker Ontology | Not Available |
|---|
| VMH ID | IM4AC |
|---|
| MarkerDB ID | Not Available |
|---|
| Good Scents ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Madsen Christian; Jensen Anders A; Liljefors Tommy; Kristiansen Uffe; Nielsen Birgitte; Hansen Camilla P; Larsen Mogens; Ebert Bjarke; Bang-Andersen Benny; Krogsgaard-Larsen Povl; Frolund Bente 5-Substituted imidazole-4-acetic acid analogues: synthesis, modeling, and pharmacological characterization of a series of novel gamma-aminobutyric acid(C) receptor agonists. Journal of medicinal chemistry (2007), 50(17), 4147-61. |
|---|
| Material Safety Data Sheet (MSDS) | Download (PDF) |
|---|
| General References | - Khandelwal JK, Prell GD, Morrishow AM, Green JP: Presence and measurement of imidazoleacetic acid, a gamma-aminobutyric acid agonist, in rat brain and human cerebrospinal fluid. J Neurochem. 1989 Apr;52(4):1107-13. [PubMed:2926392 ]
- Tsuruta Y, Tomida H, Kohashi K, Ohkura Y: Simultaneous determination of imidazoleacetic acid and N tau- and N pi-methylimidazoleacetic acids in human urine by high-performance liquid chromatography with fluorescence detection. J Chromatogr. 1987 Apr 24;416(1):63-9. [PubMed:3597642 ]
- Khandelwal JK, Hough LB, Pazhenchevsky B, Morrishow AM, Green JP: Presence and measurement of methylimidazoleacetic acids in brain and body fluids. J Biol Chem. 1982 Nov 10;257(21):12815-9. [PubMed:7130180 ]
- Sondergaard I: Quantitative determination of 1,4-methyl-imidazoleacetic acid in urine by high performance liquid chromatography. Allergy. 1982 Nov;37(8):581-6. [PubMed:7181054 ]
- Imamura I, Watanabe T, Hase Y, Sakamoto Y, Fukuda Y, Yamamoto H, Tsuruhara T, Wada H: Histamine metabolism in patients with histidinemia: determination of urinary levels of histamine, N tau-methylhistamine, imidazole acetic acid, and its conjugate(s). J Biochem. 1984 Dec;96(6):1925-9. [PubMed:6530403 ]
- Thiele I, Swainston N, Fleming RM, Hoppe A, Sahoo S, Aurich MK, Haraldsdottir H, Mo ML, Rolfsson O, Stobbe MD, Thorleifsson SG, Agren R, Bolling C, Bordel S, Chavali AK, Dobson P, Dunn WB, Endler L, Hala D, Hucka M, Hull D, Jameson D, Jamshidi N, Jonsson JJ, Juty N, Keating S, Nookaew I, Le Novere N, Malys N, Mazein A, Papin JA, Price ND, Selkov E Sr, Sigurdsson MI, Simeonidis E, Sonnenschein N, Smallbone K, Sorokin A, van Beek JH, Weichart D, Goryanin I, Nielsen J, Westerhoff HV, Kell DB, Mendes P, Palsson BO: A community-driven global reconstruction of human metabolism. Nat Biotechnol. 2013 May;31(5):419-25. doi: 10.1038/nbt.2488. Epub 2013 Mar 3. [PubMed:23455439 ]
- Elshenawy S, Pinney SE, Stuart T, Doulias PT, Zura G, Parry S, Elovitz MA, Bennett MJ, Bansal A, Strauss JF 3rd, Ischiropoulos H, Simmons RA: The Metabolomic Signature of the Placenta in Spontaneous Preterm Birth. Int J Mol Sci. 2020 Feb 4;21(3). pii: ijms21031043. doi: 10.3390/ijms21031043. [PubMed:32033212 ]
|
|---|