| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Expected but not Quantified |
|---|
| Creation Date | 2009-04-06 16:19:43 UTC |
|---|
| Update Date | 2023-02-21 17:17:38 UTC |
|---|
| HMDB ID | HMDB0012130 |
|---|
| Secondary Accession Numbers | - HMDB0059657
- HMDB0062589
- HMDB12130
- HMDB59657
- HMDB62589
|
|---|
| Metabolite Identification |
|---|
| Common Name | 2,3,4,5-Tetrahydro-2-pyridinecarboxylic acid |
|---|
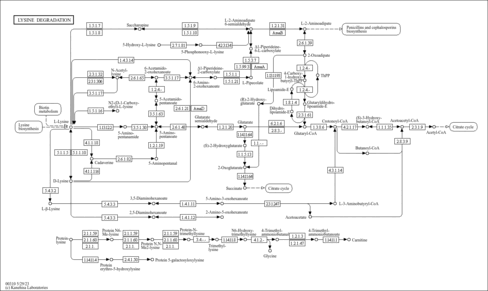
| Description | 2,3,4,5-Tetrahydro-2-pyridinecarboxylic acid (CAS: 3038-89-9), also known as 2,3,4,5-tetrahydropiperidine-2-carboxylate and 1-piperideine-6-carboxylic acid, is a cyclic intermediate in lysine degradation. L-Lysine is an essential amino acid that is a necessary building block for all protein in the body and It plays a major role in calcium absorption; building muscle protein; recovering from surgery or sports injuries; and the body's production of hormones, enzymes, and antibodies. In the lysine degradation pathway, 2,3,4,5-tetrahydro-2-pyridinecarboxylic acid is a substrate for L-aminoadipate-semialdehyde dehydrogenase (amaA) and can be formed by the spontaneous cyclization of 2-aminoadipate-6-semialdehyde. 2,3,4,5-Tetrahydro-2-pyridinecarboxylic acid is also an intermediate in glycine, serine, and threonine metabolism. It is a substrate for peroxisomal sarcosine oxidase. |
|---|
| Structure | InChI=1S/C6H9NO2/c8-6(9)5-3-1-2-4-7-5/h4-5H,1-3H2,(H,8,9)/t5-/m0/s1 |
|---|
| Synonyms | | Value | Source |
|---|
| (S)-2,3,4,5-Tetrahydropyridine-2-carboxylic acid | ChEBI | | delta1-Piperideine-6-L-carboxylate | ChEBI | | Delta(1)-Piperideine-6-L-carboxylic acid | ChEBI | | (S)-2,3,4,5-Tetrahydropyridine-2-carboxylate | Generator | | delta1-Piperideine-6-L-carboxylic acid | Generator | | Δ1-piperideine-6-L-carboxylate | Generator | | Δ1-piperideine-6-L-carboxylic acid | Generator | | delta(1)-Piperideine-6-L-carboxylate | Generator | | Δ(1)-piperideine-6-L-carboxylate | Generator | | Δ(1)-piperideine-6-L-carboxylic acid | Generator | | 2,3,4,5-Tetrahydro-2-pyridinecarboxylate | Generator | | 2,3,4,5-tetrahydro-2-pyridinecarboxylate | HMDB | | (S)-1-Piperideine-6-carboxylate | HMDB | | (2S)-2,3,4,5-Tetrahydro-2-pyridinecarboxylic acid | HMDB | | (S)-2,3,4,5-Tetrahydro-2-pyridinecarboxylic acid | HMDB | | (S)-2,3,4,5-Tetrahydropiperidine-2-carboxylate | HMDB | | (S)-2,3,4,5-Tetrahydropiperidine-2-carboxylic acid | HMDB | | 1-Piperideine-6-carboxylate | HMDB | | 1-Piperideine-6-carboxylic acid | HMDB | | 2,3,4,5-Tetrahydropicolinic acid | HMDB | | delta1-Piperideine-6-carboxylate | HMDB | | delta1-Piperideine-6-carboxylic acid | HMDB | | delta1-Piperidine-6-carboxylate | HMDB | | delta1-Piperidine-6-carboxylic acid | HMDB | | Δ1-piperidine-6-carboxylate | HMDB | | Δ1-piperidine-6-carboxylic acid | HMDB | | Δ1-piperideine-6-carboxylate | HMDB | | Δ1-piperideine-6-carboxylic acid | HMDB | | 2,3,4,5-Tetrahydro-2-pyridinecarboxylic acid | HMDB |
|
|---|
| Chemical Formula | C6H9NO2 |
|---|
| Average Molecular Weight | 127.1412 |
|---|
| Monoisotopic Molecular Weight | 127.063328537 |
|---|
| IUPAC Name | (2S)-2,3,4,5-tetrahydropyridine-2-carboxylic acid |
|---|
| Traditional Name | (2S)-2,3,4,5-tetrahydropyridine-2-carboxylic acid |
|---|
| CAS Registry Number | 73980-78-6 |
|---|
| SMILES | OC(=O)[C@@H]1CCCC=N1 |
|---|
| InChI Identifier | InChI=1S/C6H9NO2/c8-6(9)5-3-1-2-4-7-5/h4-5H,1-3H2,(H,8,9)/t5-/m0/s1 |
|---|
| InChI Key | CSDPVAKVEWETFG-YFKPBYRVSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as alpha amino acids and derivatives. These are amino acids in which the amino group is attached to the carbon atom immediately adjacent to the carboxylate group (alpha carbon), or a derivative thereof. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Alpha amino acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-amino acid or derivatives
- Tetrahydropyridine
- Hydropyridine
- Azacycle
- Organoheterocyclic compound
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Organic oxide
- Hydrocarbon derivative
- Organic oxygen compound
- Organooxygen compound
- Organonitrogen compound
- Imine
- Carbonyl group
- Organic nitrogen compound
- Organopnictogen compound
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Not Available | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Not Available |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Kovats Retention IndicesUnderivatizedDerivatized| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| 2,3,4,5-Tetrahydro-2-pyridinecarboxylic acid,1TMS,isomer #1 | C[Si](C)(C)OC(=O)[C@@H]1CCCC=N1 | 1226.7 | Semi standard non polar | 33892256 | | 2,3,4,5-Tetrahydro-2-pyridinecarboxylic acid,1TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)[C@@H]1CCCC=N1 | 1444.8 | Semi standard non polar | 33892256 |
|
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - 2,3,4,5-Tetrahydro-2-pyridinecarboxylic acid GC-MS (Non-derivatized) - 70eV, Positive | splash10-0f9x-9000000000-7bc5d56fa658fac7e9e0 | 2017-09-20 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 2,3,4,5-Tetrahydro-2-pyridinecarboxylic acid GC-MS (1 TMS) - 70eV, Positive | splash10-001i-9100000000-26047328d0a1182e46ed | 2017-10-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 2,3,4,5-Tetrahydro-2-pyridinecarboxylic acid GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 2,3,4,5-Tetrahydro-2-pyridinecarboxylic acid 10V, Positive-QTOF | splash10-01t9-3900000000-7acf87e49673fc08eeaa | 2017-10-06 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 2,3,4,5-Tetrahydro-2-pyridinecarboxylic acid 20V, Positive-QTOF | splash10-001i-9400000000-6ae0da8e10e93ebebb84 | 2017-10-06 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 2,3,4,5-Tetrahydro-2-pyridinecarboxylic acid 40V, Positive-QTOF | splash10-0f8c-9000000000-a147c4de8fc31bccca5d | 2017-10-06 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 2,3,4,5-Tetrahydro-2-pyridinecarboxylic acid 10V, Negative-QTOF | splash10-004i-2900000000-f6e66950933f111873a1 | 2017-10-06 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 2,3,4,5-Tetrahydro-2-pyridinecarboxylic acid 20V, Negative-QTOF | splash10-003r-7900000000-c03c466dc61aeb1bfe71 | 2017-10-06 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 2,3,4,5-Tetrahydro-2-pyridinecarboxylic acid 40V, Negative-QTOF | splash10-001i-9000000000-45b9069abf74b9d12e30 | 2017-10-06 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 2,3,4,5-Tetrahydro-2-pyridinecarboxylic acid 10V, Positive-QTOF | splash10-001i-9200000000-cbf1b8e349aa6ca99662 | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 2,3,4,5-Tetrahydro-2-pyridinecarboxylic acid 20V, Positive-QTOF | splash10-001i-9000000000-29fae23b5d3c449f94d6 | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 2,3,4,5-Tetrahydro-2-pyridinecarboxylic acid 40V, Positive-QTOF | splash10-001i-9000000000-56e545e596bbc86defd6 | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 2,3,4,5-Tetrahydro-2-pyridinecarboxylic acid 10V, Negative-QTOF | splash10-004i-0900000000-f5ad3a146c0d7eb97e47 | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 2,3,4,5-Tetrahydro-2-pyridinecarboxylic acid 20V, Negative-QTOF | splash10-0059-4900000000-bd8c2d97a8df0a7c74c9 | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 2,3,4,5-Tetrahydro-2-pyridinecarboxylic acid 40V, Negative-QTOF | splash10-000x-9000000000-4ee1f563852d8b5ca9f3 | 2021-09-25 | Wishart Lab | View Spectrum |
NMR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum |
IR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M-H]-) | 2023-02-03 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M+H]+) | 2023-02-03 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M+Na]+) | 2023-02-03 | FELIX lab | View Spectrum |
|
|---|