| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Expected but not Quantified |
|---|
| Creation Date | 2009-06-18 12:13:05 UTC |
|---|
| Update Date | 2021-09-14 15:46:22 UTC |
|---|
| HMDB ID | HMDB0012451 |
|---|
| Secondary Accession Numbers | - HMDB0060093
- HMDB12451
- HMDB60093
|
|---|
| Metabolite Identification |
|---|
| Common Name | all-trans-5,6-Epoxyretinoic acid |
|---|
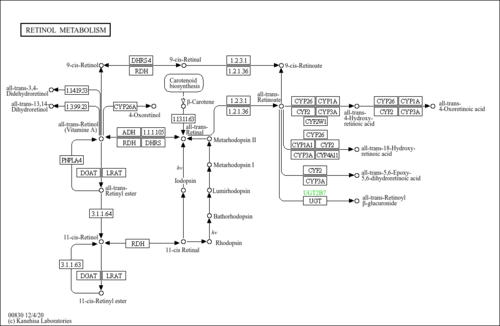
| Description | all-trans-5,6-Epoxyretinoic acid, also known as 5,6-epoxy-atRA, is classified as a member of the retinoids. Retinoids are oxygenated derivatives of 3,7-dimethyl-1-(2,6,6-trimethylcyclohex-1-enyl)nona-1,3,5,7-tetraene and derivatives thereof. all-trans-5,6-Epoxyretinoic acid is considered to be a practically insoluble (in water) and a weak acidic compound. all-trans-5,6-Epoxyretinoic acid is an isoprenoid lipid molecule. all-trans-5,6-Epoxyretinoic acid can be found primarily in human kidney and liver tissues; and in blood and urine. Within a cell, all-trans-5,6-epoxyretinoic acid is primarily located in the cytoplasm, in the extracellular space, or near the membrane. |
|---|
| Structure | C\C(\C=C\C12OC1(C)CCCC2(C)C)=C/C=C/C(/C)=C/C(O)=O InChI=1S/C20H28O3/c1-15(8-6-9-16(2)14-17(21)22)10-13-20-18(3,4)11-7-12-19(20,5)23-20/h6,8-10,13-14H,7,11-12H2,1-5H3,(H,21,22)/b9-6+,13-10+,15-8+,16-14+ |
|---|
| Synonyms | | Value | Source |
|---|
| all-trans-5,6-Epoxy-5,6-dihydroretinoic acid | ChEBI | | all-trans-5,6-Epoxy-5,6-dihydroretinoate | Generator | | all-trans-5,6-Epoxyretinoate | Generator | | 5,6-Epoxy-5,6-dihydro-retinoate | HMDB | | 5,6-Epoxy-5,6-dihydro-retinoic acid | HMDB | | 5,6-Epoxyretinoic acid, (13-cis)-isomer | MeSH, HMDB | | 5,6-Epoxyretinoic acid, sodium salt | MeSH, HMDB | | 5,6-Epoxyretinoic acid | MeSH, HMDB | | 5,6-Epoxy-5,6-dihydroretinoic acid | HMDB | | 5,6-Epoxy-atRA | HMDB | | all-trans-5,6-Epoxyretinoic acid | HMDB |
|
|---|
| Chemical Formula | C20H28O3 |
|---|
| Average Molecular Weight | 316.4345 |
|---|
| Monoisotopic Molecular Weight | 316.203844762 |
|---|
| IUPAC Name | (2E,4E,6E,8E)-3,7-dimethyl-9-{2,2,6-trimethyl-7-oxabicyclo[4.1.0]heptan-1-yl}nona-2,4,6,8-tetraenoic acid |
|---|
| Traditional Name | (2E,4E,6E,8E)-3,7-dimethyl-9-{2,2,6-trimethyl-7-oxabicyclo[4.1.0]heptan-1-yl}nona-2,4,6,8-tetraenoic acid |
|---|
| CAS Registry Number | 13100-69-1 |
|---|
| SMILES | C\C(\C=C\C12OC1(C)CCCC2(C)C)=C/C=C/C(/C)=C/C(O)=O |
|---|
| InChI Identifier | InChI=1S/C20H28O3/c1-15(8-6-9-16(2)14-17(21)22)10-13-20-18(3,4)11-7-12-19(20,5)23-20/h6,8-10,13-14H,7,11-12H2,1-5H3,(H,21,22)/b9-6+,13-10+,15-8+,16-14+ |
|---|
| InChI Key | KEEHJLBAOLGBJZ-WEDZBJJJSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as retinoids. These are oxygenated derivatives of 3,7-dimethyl-1-(2,6,6-trimethylcyclohex-1-enyl)nona-1,3,5,7-tetraene and derivatives thereof. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Retinoids |
|---|
| Direct Parent | Retinoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Retinoic acid
- Diterpenoid
- Retinoid skeleton
- Medium-chain fatty acid
- Branched fatty acid
- Epoxy fatty acid
- Heterocyclic fatty acid
- Oxepane
- Methyl-branched fatty acid
- Unsaturated fatty acid
- Fatty acyl
- Fatty acid
- Carboxylic acid derivative
- Organoheterocyclic compound
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Ether
- Oxacycle
- Oxirane
- Dialkyl ether
- Hydrocarbon derivative
- Organic oxygen compound
- Carbonyl group
- Organooxygen compound
- Organic oxide
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Physiological effect | Not Available |
|---|
| Disposition | |
|---|
| Process | Not Available |
|---|
| Role | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Not Available |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Kovats Retention IndicesUnderivatizedDerivatized |
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - all-trans-5,6-Epoxyretinoic acid GC-MS (Non-derivatized) - 70eV, Positive | splash10-0gb9-9174000000-06301725f04dbf9da5b0 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - all-trans-5,6-Epoxyretinoic acid GC-MS (1 TMS) - 70eV, Positive | splash10-00di-9126000000-aaab7da3a75ae7d0ac69 | 2017-10-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - all-trans-5,6-Epoxyretinoic acid GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - all-trans-5,6-Epoxyretinoic acid 10V, Positive-QTOF | splash10-066s-0394000000-5ab9d9f9a6f2942b53fe | 2016-08-03 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - all-trans-5,6-Epoxyretinoic acid 20V, Positive-QTOF | splash10-0a4i-3490000000-ecf50862a1162e1b0ba8 | 2016-08-03 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - all-trans-5,6-Epoxyretinoic acid 40V, Positive-QTOF | splash10-0a4i-9300000000-801580c3c0c49c76fd08 | 2016-08-03 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - all-trans-5,6-Epoxyretinoic acid 10V, Negative-QTOF | splash10-014i-0179000000-815f6a20a086d254820b | 2016-08-03 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - all-trans-5,6-Epoxyretinoic acid 20V, Negative-QTOF | splash10-01b9-1195000000-c470ae42b6bb7613d063 | 2016-08-03 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - all-trans-5,6-Epoxyretinoic acid 40V, Negative-QTOF | splash10-05a9-6960000000-e311a77eab0f7eeff493 | 2016-08-03 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - all-trans-5,6-Epoxyretinoic acid 10V, Positive-QTOF | splash10-00rt-0192000000-861041c7dc974b088a42 | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - all-trans-5,6-Epoxyretinoic acid 20V, Positive-QTOF | splash10-0540-0590000000-4f77ff24993914b2af45 | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - all-trans-5,6-Epoxyretinoic acid 40V, Positive-QTOF | splash10-0gdu-7910000000-3b294bfc029780e1a389 | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - all-trans-5,6-Epoxyretinoic acid 10V, Negative-QTOF | splash10-01b9-0198000000-91d1b99148247a6bd981 | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - all-trans-5,6-Epoxyretinoic acid 20V, Negative-QTOF | splash10-00y0-0971000000-29094eb01d6c33d9ba2e | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - all-trans-5,6-Epoxyretinoic acid 40V, Negative-QTOF | splash10-066r-2932000000-86038457c94d4ed88c27 | 2021-09-25 | Wishart Lab | View Spectrum |
|
|---|
| General References | - Thiele I, Swainston N, Fleming RM, Hoppe A, Sahoo S, Aurich MK, Haraldsdottir H, Mo ML, Rolfsson O, Stobbe MD, Thorleifsson SG, Agren R, Bolling C, Bordel S, Chavali AK, Dobson P, Dunn WB, Endler L, Hala D, Hucka M, Hull D, Jameson D, Jamshidi N, Jonsson JJ, Juty N, Keating S, Nookaew I, Le Novere N, Malys N, Mazein A, Papin JA, Price ND, Selkov E Sr, Sigurdsson MI, Simeonidis E, Sonnenschein N, Smallbone K, Sorokin A, van Beek JH, Weichart D, Goryanin I, Nielsen J, Westerhoff HV, Kell DB, Mendes P, Palsson BO: A community-driven global reconstruction of human metabolism. Nat Biotechnol. 2013 May;31(5):419-25. doi: 10.1038/nbt.2488. Epub 2013 Mar 3. [PubMed:23455439 ]
- Simons K, Toomre D: Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000 Oct;1(1):31-9. [PubMed:11413487 ]
- Watson AD: Thematic review series: systems biology approaches to metabolic and cardiovascular disorders. Lipidomics: a global approach to lipid analysis in biological systems. J Lipid Res. 2006 Oct;47(10):2101-11. Epub 2006 Aug 10. [PubMed:16902246 ]
- Sethi JK, Vidal-Puig AJ: Thematic review series: adipocyte biology. Adipose tissue function and plasticity orchestrate nutritional adaptation. J Lipid Res. 2007 Jun;48(6):1253-62. Epub 2007 Mar 20. [PubMed:17374880 ]
- Lingwood D, Simons K: Lipid rafts as a membrane-organizing principle. Science. 2010 Jan 1;327(5961):46-50. doi: 10.1126/science.1174621. [PubMed:20044567 ]
- Gunstone, Frank D., John L. Harwood, and Albert J. Dijkstra (2007). The lipid handbook with CD-ROM. CRC Press.
|
|---|