| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Detected and Quantified |
|---|
| Creation Date | 2005-11-16 15:48:42 UTC |
|---|
| Update Date | 2021-09-14 15:40:10 UTC |
|---|
| HMDB ID | HMDB0000174 |
|---|
| Secondary Accession Numbers | - HMDB00174
- HMDB0059624
- HMDB59624
|
|---|
| Metabolite Identification |
|---|
| Common Name | L-Fucose |
|---|
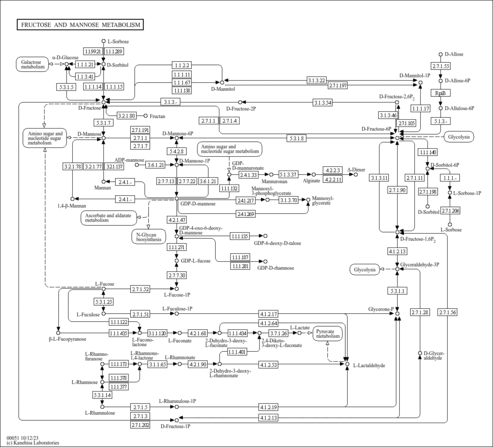
| Description | Fucose (CAS: 2438-80-4) is a hexose deoxy sugar with the chemical formula C6H12O5. L-Fucose (6-deoxy-L-galactose) is a monosaccharide that is a common component of many N- and O-linked glycans and glycolipids produced by mammalian cells. It is the fundamental subunit of the fucoidan polysaccharide. As a free sugar, L-fucose is normally found at very low levels in mammals. It is unique in that it is the only levorotatory sugar synthesized and utilized by mammals. Fucose polymers are synthesized by fucosyltransferases. All fucosyltransferases utilize a nucleotide-activated form of fucose, GDP-fucose, as a fucose donor in the construction of fucosylated oligosaccharides. The ABO blood group antigens are among the most well known fucosylated glycans. The alpha-1->3 linked core fucose is a suspected carbohydrate antigen for IgE-mediated allergy. Two structural features distinguish fucose from other six-carbon sugars present in mammals: the lack of a hydroxyl group on the carbon at the 6-position (C-6) and the L-configuration. In fucose-containing glycan structures, fucosylated glycans, fucose can exist as a terminal modification or serve as an attachment point for adding other sugars. Fucose is metabolized by an enzyme called alpha-fucosidase. Fucose is secreted in urine when the liver is damaged. Free L-fucose in serum and urine can be used as a marker for cancer, cirrhosis, alcoholic liver disease and gastric ulcers (PMID: 2311216 , 8488966 ). Elevated levels of serum fucose have been reported in breast cancer, ovarian cancer, lung cancer, liver cancer, diabetes, and cardiovascular disease. It has been shown that feeding rats a diet high in L-fucose induces neuropathy similar to that seen in diabetics. |
|---|
| Structure | C[C@@H]1O[C@@H](O)[C@@H](O)[C@H](O)[C@@H]1O InChI=1S/C6H12O5/c1-2-3(7)4(8)5(9)6(10)11-2/h2-10H,1H3/t2-,3+,4+,5-,6+/m0/s1 |
|---|
| Synonyms | | Value | Source |
|---|
| alpha-L-Fuc | ChEBI | | alpha-L-Fucp | ChEBI | | a-L-Fuc | Generator | | α-L-Fuc | Generator | | a-L-Fucose | Generator | | α-L-fucose | Generator | | a-L-Fucp | Generator | | α-L-fucp | Generator | | 6-Deoxy-L-galactopyranose | HMDB | | 6-Deoxy-L-galactose | HMDB | | 6-Deoxy-alpha-L-galactopyranose | HMDB | | 6-Deoxy-alpha-L-galactose | HMDB | | 6-Deoxy-α-L-galactopyranose | HMDB | | 6-Deoxy-α-L-galactose | HMDB | | alpha-L-Fucopyranose | HMDB | | alpha-L-Fucose | HMDB | | α-L-Fucopyranose | HMDB | | α-L-Fucose | HMDB | | (-)-Fucose | HMDB | | 6-Desoxygalactose | HMDB | | Fucose | HMDB | | L-(-)-Fucose | HMDB | | L-Fucose | HMDB | | L-Galactomethylose | HMDB |
|
|---|
| Chemical Formula | C6H12O5 |
|---|
| Average Molecular Weight | 164.1565 |
|---|
| Monoisotopic Molecular Weight | 164.068473494 |
|---|
| IUPAC Name | (2R,3S,4R,5S,6S)-6-methyloxane-2,3,4,5-tetrol |
|---|
| Traditional Name | α-L-fucose |
|---|
| CAS Registry Number | 6696-41-9 |
|---|
| SMILES | C[C@@H]1O[C@@H](O)[C@@H](O)[C@H](O)[C@@H]1O |
|---|
| InChI Identifier | InChI=1S/C6H12O5/c1-2-3(7)4(8)5(9)6(10)11-2/h2-10H,1H3/t2-,3+,4+,5-,6+/m0/s1 |
|---|
| InChI Key | SHZGCJCMOBCMKK-SXUWKVJYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as hexoses. These are monosaccharides in which the sugar unit is a is a six-carbon containing moeity. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Hexoses |
|---|
| Alternative Parents | |
|---|
| Substituents | - Hexose monosaccharide
- Oxane
- Secondary alcohol
- Hemiacetal
- Oxacycle
- Organoheterocyclic compound
- Polyol
- Hydrocarbon derivative
- Alcohol
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Physiological effect | Not Available |
|---|
| Disposition | |
|---|
| Process | |
|---|
| Role | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | 140 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 985 mg/mL | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Not Available |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Retention Times Underivatized| Chromatographic Method | Retention Time | Reference |
|---|
| Measured using a Waters Acquity ultraperformance liquid chromatography (UPLC) ethylene-bridged hybrid (BEH) C18 column (100 mm × 2.1 mm; 1.7 μmparticle diameter). Predicted by Afia on May 17, 2022. Predicted by Afia on May 17, 2022. | 0.79 minutes | 32390414 | | Predicted by Siyang on May 30, 2022 | 9.7099 minutes | 33406817 | | Predicted by Siyang using ReTip algorithm on June 8, 2022 | 3.04 minutes | 32390414 | | AjsUoB = Accucore 150 Amide HILIC with 10mM Ammonium Formate, 0.1% Formic Acid | 245.5 seconds | 40023050 |
Predicted Kovats Retention IndicesUnderivatizedDerivatized| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| L-Fucose,1TMS,isomer #1 | C[C@@H]1O[C@@H](O[Si](C)(C)C)[C@@H](O)[C@H](O)[C@@H]1O | 1458.1 | Semi standard non polar | 33892256 | | L-Fucose,1TMS,isomer #2 | C[C@@H]1O[C@@H](O)[C@@H](O[Si](C)(C)C)[C@H](O)[C@@H]1O | 1468.2 | Semi standard non polar | 33892256 | | L-Fucose,1TMS,isomer #3 | C[C@@H]1O[C@@H](O)[C@@H](O)[C@H](O[Si](C)(C)C)[C@@H]1O | 1481.0 | Semi standard non polar | 33892256 | | L-Fucose,1TMS,isomer #4 | C[C@@H]1O[C@@H](O)[C@@H](O)[C@H](O)[C@@H]1O[Si](C)(C)C | 1496.0 | Semi standard non polar | 33892256 | | L-Fucose,2TMS,isomer #1 | C[C@@H]1O[C@@H](O[Si](C)(C)C)[C@@H](O[Si](C)(C)C)[C@H](O)[C@@H]1O | 1495.4 | Semi standard non polar | 33892256 | | L-Fucose,2TMS,isomer #2 | C[C@@H]1O[C@@H](O[Si](C)(C)C)[C@@H](O)[C@H](O[Si](C)(C)C)[C@@H]1O | 1529.5 | Semi standard non polar | 33892256 | | L-Fucose,2TMS,isomer #3 | C[C@@H]1O[C@@H](O[Si](C)(C)C)[C@@H](O)[C@H](O)[C@@H]1O[Si](C)(C)C | 1514.2 | Semi standard non polar | 33892256 | | L-Fucose,2TMS,isomer #4 | C[C@@H]1O[C@@H](O)[C@@H](O[Si](C)(C)C)[C@H](O[Si](C)(C)C)[C@@H]1O | 1523.2 | Semi standard non polar | 33892256 | | L-Fucose,2TMS,isomer #5 | C[C@@H]1O[C@@H](O)[C@@H](O[Si](C)(C)C)[C@H](O)[C@@H]1O[Si](C)(C)C | 1544.2 | Semi standard non polar | 33892256 | | L-Fucose,2TMS,isomer #6 | C[C@@H]1O[C@@H](O)[C@@H](O)[C@H](O[Si](C)(C)C)[C@@H]1O[Si](C)(C)C | 1504.2 | Semi standard non polar | 33892256 | | L-Fucose,3TMS,isomer #1 | C[C@@H]1O[C@@H](O[Si](C)(C)C)[C@@H](O[Si](C)(C)C)[C@H](O[Si](C)(C)C)[C@@H]1O | 1572.7 | Semi standard non polar | 33892256 | | L-Fucose,3TMS,isomer #2 | C[C@@H]1O[C@@H](O[Si](C)(C)C)[C@@H](O[Si](C)(C)C)[C@H](O)[C@@H]1O[Si](C)(C)C | 1582.5 | Semi standard non polar | 33892256 | | L-Fucose,3TMS,isomer #3 | C[C@@H]1O[C@@H](O[Si](C)(C)C)[C@@H](O)[C@H](O[Si](C)(C)C)[C@@H]1O[Si](C)(C)C | 1589.1 | Semi standard non polar | 33892256 | | L-Fucose,3TMS,isomer #4 | C[C@@H]1O[C@@H](O)[C@@H](O[Si](C)(C)C)[C@H](O[Si](C)(C)C)[C@@H]1O[Si](C)(C)C | 1581.2 | Semi standard non polar | 33892256 | | L-Fucose,4TMS,isomer #1 | C[C@@H]1O[C@@H](O[Si](C)(C)C)[C@@H](O[Si](C)(C)C)[C@H](O[Si](C)(C)C)[C@@H]1O[Si](C)(C)C | 1681.3 | Semi standard non polar | 33892256 | | L-Fucose,1TBDMS,isomer #1 | C[C@@H]1O[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H](O)[C@H](O)[C@@H]1O | 1720.3 | Semi standard non polar | 33892256 | | L-Fucose,1TBDMS,isomer #2 | C[C@@H]1O[C@@H](O)[C@@H](O[Si](C)(C)C(C)(C)C)[C@H](O)[C@@H]1O | 1741.2 | Semi standard non polar | 33892256 | | L-Fucose,1TBDMS,isomer #3 | C[C@@H]1O[C@@H](O)[C@@H](O)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O | 1741.1 | Semi standard non polar | 33892256 | | L-Fucose,1TBDMS,isomer #4 | C[C@@H]1O[C@@H](O)[C@@H](O)[C@H](O)[C@@H]1O[Si](C)(C)C(C)(C)C | 1757.7 | Semi standard non polar | 33892256 | | L-Fucose,2TBDMS,isomer #1 | C[C@@H]1O[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H](O[Si](C)(C)C(C)(C)C)[C@H](O)[C@@H]1O | 1987.4 | Semi standard non polar | 33892256 | | L-Fucose,2TBDMS,isomer #2 | C[C@@H]1O[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H](O)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O | 2014.5 | Semi standard non polar | 33892256 | | L-Fucose,2TBDMS,isomer #3 | C[C@@H]1O[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H](O)[C@H](O)[C@@H]1O[Si](C)(C)C(C)(C)C | 2016.9 | Semi standard non polar | 33892256 | | L-Fucose,2TBDMS,isomer #4 | C[C@@H]1O[C@@H](O)[C@@H](O[Si](C)(C)C(C)(C)C)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O | 2008.0 | Semi standard non polar | 33892256 | | L-Fucose,2TBDMS,isomer #5 | C[C@@H]1O[C@@H](O)[C@@H](O[Si](C)(C)C(C)(C)C)[C@H](O)[C@@H]1O[Si](C)(C)C(C)(C)C | 2029.2 | Semi standard non polar | 33892256 | | L-Fucose,2TBDMS,isomer #6 | C[C@@H]1O[C@@H](O)[C@@H](O)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O[Si](C)(C)C(C)(C)C | 2003.6 | Semi standard non polar | 33892256 | | L-Fucose,3TBDMS,isomer #1 | C[C@@H]1O[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H](O[Si](C)(C)C(C)(C)C)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O | 2255.5 | Semi standard non polar | 33892256 | | L-Fucose,3TBDMS,isomer #2 | C[C@@H]1O[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H](O[Si](C)(C)C(C)(C)C)[C@H](O)[C@@H]1O[Si](C)(C)C(C)(C)C | 2271.2 | Semi standard non polar | 33892256 | | L-Fucose,3TBDMS,isomer #3 | C[C@@H]1O[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H](O)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O[Si](C)(C)C(C)(C)C | 2269.8 | Semi standard non polar | 33892256 | | L-Fucose,3TBDMS,isomer #4 | C[C@@H]1O[C@@H](O)[C@@H](O[Si](C)(C)C(C)(C)C)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O[Si](C)(C)C(C)(C)C | 2274.9 | Semi standard non polar | 33892256 | | L-Fucose,4TBDMS,isomer #1 | C[C@@H]1O[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H](O[Si](C)(C)C(C)(C)C)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O[Si](C)(C)C(C)(C)C | 2479.4 | Semi standard non polar | 33892256 |
|
|---|
| Disease References | | Thyroid cancer |
|---|
- Sakai T, Yamamoto K, Yokota H, Hakozaki-Usui K, Hino F, Kato I: Rapid, simple enzymatic assay of free L-fucose in serum and urine, and its use as a marker for cancer, cirrhosis, and gastric ulcers. Clin Chem. 1990 Mar;36(3):474-6. [PubMed:2311216 ]
| | Colorectal cancer |
|---|
- Brown DG, Rao S, Weir TL, O'Malia J, Bazan M, Brown RJ, Ryan EP: Metabolomics and metabolic pathway networks from human colorectal cancers, adjacent mucosa, and stool. Cancer Metab. 2016 Jun 6;4:11. doi: 10.1186/s40170-016-0151-y. eCollection 2016. [PubMed:27275383 ]
- Goedert JJ, Sampson JN, Moore SC, Xiao Q, Xiong X, Hayes RB, Ahn J, Shi J, Sinha R: Fecal metabolomics: assay performance and association with colorectal cancer. Carcinogenesis. 2014 Sep;35(9):2089-96. doi: 10.1093/carcin/bgu131. Epub 2014 Jul 18. [PubMed:25037050 ]
| | Eosinophilic esophagitis |
|---|
- Slae, M., Huynh, H., Wishart, D.S. (2014). Analysis of 30 normal pediatric urine samples via NMR spectroscopy (unpublished work). NA.
|
|
|---|
| General References | - Nicholson JK, Foxall PJ, Spraul M, Farrant RD, Lindon JC: 750 MHz 1H and 1H-13C NMR spectroscopy of human blood plasma. Anal Chem. 1995 Mar 1;67(5):793-811. [PubMed:7762816 ]
- Nakagawa F, Schulte BA, Spicer SS: Lectin cytochemical evaluation of somatosensory neurons and their peripheral and central processes in rat and man. Cell Tissue Res. 1986;245(3):579-89. [PubMed:3757018 ]
- Condaminet B, Peguet-Navarro J, Stahl PD, Dalbiez-Gauthier C, Schmitt D, Berthier-Vergnes O: Human epidermal Langerhans cells express the mannose-fucose binding receptor. Eur J Immunol. 1998 Nov;28(11):3541-51. [PubMed:9842897 ]
- Havenaar EC, Hoff RC, van den Eijnden DH, van Dijk W: Sialyl Lewis(x) epitopes do not occur on acute phase proteins in mice: relationship to the absence of alpha3-fucosyltransferase in the liver. Glycoconj J. 1998 Apr;15(4):389-95. [PubMed:9613826 ]
- Roelfzema H, van Erp PE: Studies on the plasma membrane of normal and psoriatic keratinocytes. 6. Cell surface and shed glycoproteins. J Invest Dermatol. 1983 Jan;80(1):24-6. [PubMed:6184420 ]
- Wiese TJ, Dunlap JA, Yorek MA: Effect of L-fucose and D-glucose concentration on L-fucoprotein metabolism in human Hep G2 cells and changes in fucosyltransferase and alpha-L-fucosidase activity in liver of diabetic rats. Biochim Biophys Acta. 1997 Apr 17;1335(1-2):61-72. [PubMed:9133643 ]
- Isnard N, Fodil-Bourahla I, Robert AM, Robert L: Pharmacology of skin aging. Stimulation of glycosaminoglycan biosynthesis by L-fucose and fucose-rich polysaccharides, effect of in vitro aging of fibroblasts. Biomed Pharmacother. 2004 Apr;58(3):202-4. [PubMed:15082343 ]
- Rizzi M, Tonetti M, Vigevani P, Sturla L, Bisso A, Flora AD, Bordo D, Bolognesi M: GDP-4-keto-6-deoxy-D-mannose epimerase/reductase from Escherichia coli, a key enzyme in the biosynthesis of GDP-L-fucose, displays the structural characteristics of the RED protein homology superfamily. Structure. 1998 Nov 15;6(11):1453-65. [PubMed:9817848 ]
- Gheri G, Sgambati E, Thyrion GD, Vichi D, Orlandini GE: The oligosaccharidic content of the glycoconjugates of the prepubertal descended and undescended testis: lectin histochemical study. Ital J Anat Embryol. 2004 Apr-Jun;109(2):69-84. [PubMed:15481156 ]
- Fediv OI, Kolomoiets' MIu: [Role of cytokines in the metabolism of the extracellular matrix carbohydrate-protein components in patients of various ages with gastroduodenal ulcer]. Lik Sprava. 2001 Jul-Aug;(4):181-2. [PubMed:11692712 ]
- Hutchinson WL, Du MQ, Johnson PJ, Williams R: Fucosyltransferases: differential plasma and tissue alterations in hepatocellular carcinoma and cirrhosis. Hepatology. 1991 Apr;13(4):683-8. [PubMed:1849114 ]
- Fleischer M, Przondo-Mordarska A: [Adhesins of Acinetobacter strains]. Med Dosw Mikrobiol. 1998;50(3-4):229-37. [PubMed:10222738 ]
- Gohlke M, Baude G, Nuck R, Grunow D, Kannicht C, Bringmann P, Donner P, Reutter W: O-linked L-fucose is present in Desmodus rotundus salivary plasminogen activator. J Biol Chem. 1996 Mar 29;271(13):7381-6. [PubMed:8631761 ]
- Chien CM, Cheng JL, Chang WT, Tien MH, Tsao CM, Chang YH, Chang HY, Hsieh JF, Wong CH, Chen ST: Polysaccharides of Ganoderma lucidum alter cell immunophenotypic expression and enhance CD56+ NK-cell cytotoxicity in cord blood. Bioorg Med Chem. 2004 Nov 1;12(21):5603-9. [PubMed:15465338 ]
- Hanisch FG, Heimbuchel G, Baldus SE, Uhlenbruck G, Schmits R, Pfreundschuh M, Schwonzen M, Vierbuchen M, Bara J, Peter-Katalinic J: Monoclonal antibody FW6 defines an epitope on alpha 3/4-monofucosylated polylactosaminoglycans expressed by fetal and colon carcinoma-associated mucins. Cancer Res. 1993 Sep 15;53(18):4367-75. [PubMed:8364932 ]
- Tonetti M, Sturla L, Bisso A, Zanardi D, Benatti U, De Flora A: The metabolism of 6-deoxyhexoses in bacterial and animal cells. Biochimie. 1998 Nov;80(11):923-31. [PubMed:9893952 ]
- Sato T, Sato M, Kiyohara K, Sogabe M, Shikanai T, Kikuchi N, Togayachi A, Ishida H, Ito H, Kameyama A, Gotoh M, Narimatsu H: Molecular cloning and characterization of a novel human beta1,3-glucosyltransferase, which is localized at the endoplasmic reticulum and glucosylates O-linked fucosylglycan on thrombospondin type 1 repeat domain. Glycobiology. 2006 Dec;16(12):1194-206. Epub 2006 Aug 9. [PubMed:16899492 ]
- Coyne MJ, Reinap B, Lee MM, Comstock LE: Human symbionts use a host-like pathway for surface fucosylation. Science. 2005 Mar 18;307(5716):1778-81. [PubMed:15774760 ]
- Quirk S, Seley KL: Substrate discrimination by the human GTP fucose pyrophosphorylase. Biochemistry. 2005 Aug 16;44(32):10854-63. [PubMed:16086588 ]
- Bathi RJ, Nandimath K, Kannan N, Shetty P: Evaluation of glycoproteins as prognosticators in head and neck malignancy. Indian J Dent Res. 2001 Apr-Jun;12(2):93-9. [PubMed:11665403 ]
- Sakai T, Yamamoto K, Yokota H, Hakozaki-Usui K, Hino F, Kato I: Rapid, simple enzymatic assay of free L-fucose in serum and urine, and its use as a marker for cancer, cirrhosis, and gastric ulcers. Clin Chem. 1990 Mar;36(3):474-6. [PubMed:2311216 ]
- Yamauchi M, Kimura K, Maezawa Y, Ohata M, Mizuhara Y, Hirakawa J, Nakajima H, Toda G: Urinary level of L-fucose as a marker of alcoholic liver disease. Alcohol Clin Exp Res. 1993 Apr;17(2):268-71. [PubMed:8488966 ]
- Chang TW, Gorbach SL, Bartlett JG: Inhibition of binding of Clostridium difficile toxin by steroids. J Infect Dis. 1980 Jul;142(1):113. [PubMed:6772711 ]
- Neethling FA, Koren E, Ye Y, Richards SV, Kujundzic M, Oriol R, Cooper DK: Protection of pig kidney (PK15) cells from the cytotoxic effect of anti-pig antibodies by alpha-galactosyl oligosaccharides. Transplantation. 1994 Mar 27;57(6):959-63. [PubMed:8154046 ]
|
|---|