| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Detected and Quantified |
|---|
| Creation Date | 2006-08-16 14:44:11 UTC |
|---|
| Update Date | 2022-03-07 02:49:09 UTC |
|---|
| HMDB ID | HMDB0001419 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 24-Hydroxycholesterol |
|---|
| Description | 24-Hydroxycholesterol (24OHC) is almost exclusively formed in the brain. The enzymatic conversion of CNS cholesterol to 24OHC, which readily crosses the blood-brain barrier, is the major pathway for brain cholesterol elimination and brain cholesterol homeostasis maintenance. The enzyme mediating this conversion has been characterized at the molecular level as cholesterol 24-hydroxylase (EC 1.14.13.98, CYP46) and is mainly located in neurons. Like other oxysterols, 24OHC is efficiently converted into normal bile acids or excreted in bile in its sulfated and glucuronidated form. Levels of 24OHC in the circulation decrease with age in infants and children. In adults, however, the levels appear to be stable. There is accumulating evidence pointing toward a potentially important link between cholesterol, beta-amyloid, and Alzheimer's disease. Patients with active demyelinating diseases had increased levels of 24OHC in cerebrospinal fluid (CSF). Patients with Alzheimer's disease have slightly increased levels of 24OHC in CSF. Patients with multiple sclerosis have a tendency to have higher levels of 24OHC during active periods. (PMID: 15061359 , 14574622 ). 24-Hydroxycholesterol has been found to accumulate in hereditary hypercholesterolemia, an inborn error of metabolism. |
|---|
| Structure | [H][C@@]1(CC[C@@]2([H])[C@]3([H])CC=C4C[C@@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CC[C@H](O)C(C)C InChI=1S/C27H46O2/c1-17(2)25(29)11-6-18(3)22-9-10-23-21-8-7-19-16-20(28)12-14-26(19,4)24(21)13-15-27(22,23)5/h7,17-18,20-25,28-29H,6,8-16H2,1-5H3/t18-,20+,21+,22-,23+,24+,25+,26+,27-/m1/s1 |
|---|
| Synonyms | | Value | Source |
|---|
| (24S)-Hydroxycholesterol | ChEBI | | 24S-Hydroxy-cholesterol | ChEBI | | Cerebrosterol | ChEBI | | Cholest-5-en-3beta,24S-diol | ChEBI | | Cholest-5-ene-3,24-diol | ChEBI | | (24S)-24-Hydroxycholesterol | Kegg | | (24S)-Cholest-5-ene-3beta,24-diol | Kegg | | Cholest-5-en-3b,24S-diol | Generator | | Cholest-5-en-3β,24S-diol | Generator | | (24S)-Cholest-5-ene-3b,24-diol | Generator | | (24S)-Cholest-5-ene-3β,24-diol | Generator | | 24(S)-Hydroxycholesterol | HMDB | | 24S-Cholest-5-ene-3b,24-diol | HMDB | | 24S-Hydroxycholesterol | HMDB | | Cerebrosterin | HMDB | | Cholest-5-ene-3b,24b-diol | HMDB |
|
|---|
| Chemical Formula | C27H46O2 |
|---|
| Average Molecular Weight | 402.6529 |
|---|
| Monoisotopic Molecular Weight | 402.349780716 |
|---|
| IUPAC Name | (1S,2R,5S,10S,11S,14R,15R)-14-[(2R,5S)-5-hydroxy-6-methylheptan-2-yl]-2,15-dimethyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadec-7-en-5-ol |
|---|
| Traditional Name | 24(S)-hydroxycholesterol |
|---|
| CAS Registry Number | 474-73-7 |
|---|
| SMILES | [H][C@@]1(CC[C@@]2([H])[C@]3([H])CC=C4C[C@@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CC[C@H](O)C(C)C |
|---|
| InChI Identifier | InChI=1S/C27H46O2/c1-17(2)25(29)11-6-18(3)22-9-10-23-21-8-7-19-16-20(28)12-14-26(19,4)24(21)13-15-27(22,23)5/h7,17-18,20-25,28-29H,6,8-16H2,1-5H3/t18-,20+,21+,22-,23+,24+,25+,26+,27-/m1/s1 |
|---|
| InChI Key | IOWMKBFJCNLRTC-XWXSNNQWSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as dihydroxy bile acids, alcohols and derivatives. Dihydroxy bile acids, alcohols and derivatives are compounds containing or derived from a bile acid or alcohol, and which bears exactly two carboxylic acid groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Bile acids, alcohols and derivatives |
|---|
| Direct Parent | Dihydroxy bile acids, alcohols and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - 24-hydroxysteroid
- Dihydroxy bile acid, alcohol, or derivatives
- 3-beta-hydroxysteroid
- 3-beta-hydroxy-delta-5-steroid
- Hydroxysteroid
- 3-hydroxysteroid
- 3-hydroxy-delta-5-steroid
- Delta-5-steroid
- Cyclic alcohol
- Secondary alcohol
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Alcohol
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Physiological effect | |
|---|
| Disposition | |
|---|
| Process | |
|---|
| Role | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Not Available |
|---|
| Predicted Molecular Properties | | Show more...
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Kovats Retention IndicesUnderivatizedDerivatized| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| 24-Hydroxycholesterol,1TMS,isomer #1 | CC(C)[C@@H](O)CC[C@@H](C)[C@H]1CC[C@H]2[C@@H]3CC=C4C[C@@H](O[Si](C)(C)C)CC[C@]4(C)[C@H]3CC[C@@]21C | 3400.9 | Semi standard non polar | 33892256 | | 24-Hydroxycholesterol,1TMS,isomer #2 | CC(C)[C@H](CC[C@@H](C)[C@H]1CC[C@H]2[C@@H]3CC=C4C[C@@H](O)CC[C@]4(C)[C@H]3CC[C@@]21C)O[Si](C)(C)C | 3376.0 | Semi standard non polar | 33892256 | | 24-Hydroxycholesterol,2TMS,isomer #1 | CC(C)[C@H](CC[C@@H](C)[C@H]1CC[C@H]2[C@@H]3CC=C4C[C@@H](O[Si](C)(C)C)CC[C@]4(C)[C@H]3CC[C@@]21C)O[Si](C)(C)C | 3407.5 | Semi standard non polar | 33892256 | | 24-Hydroxycholesterol,1TBDMS,isomer #1 | CC(C)[C@@H](O)CC[C@@H](C)[C@H]1CC[C@H]2[C@@H]3CC=C4C[C@@H](O[Si](C)(C)C(C)(C)C)CC[C@]4(C)[C@H]3CC[C@@]21C | 3618.3 | Semi standard non polar | 33892256 | | 24-Hydroxycholesterol,1TBDMS,isomer #2 | CC(C)[C@H](CC[C@@H](C)[C@H]1CC[C@H]2[C@@H]3CC=C4C[C@@H](O)CC[C@]4(C)[C@H]3CC[C@@]21C)O[Si](C)(C)C(C)(C)C | 3630.0 | Semi standard non polar | 33892256 | | 24-Hydroxycholesterol,2TBDMS,isomer #1 | CC(C)[C@H](CC[C@@H](C)[C@H]1CC[C@H]2[C@@H]3CC=C4C[C@@H](O[Si](C)(C)C(C)(C)C)CC[C@]4(C)[C@H]3CC[C@@]21C)O[Si](C)(C)C(C)(C)C | 3901.7 | Semi standard non polar | 33892256 |
| Show more...
|---|
| Spectra |
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - 24-Hydroxycholesterol GC-MS (Non-derivatized) - 70eV, Positive | splash10-0076-1009000000-ab69a48110c4082d2ca7 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 24-Hydroxycholesterol GC-MS (2 TMS) - 70eV, Positive | splash10-001i-2111290000-94668570804377216a37 | 2017-10-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 24-Hydroxycholesterol GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 24-Hydroxycholesterol 10V, Positive-QTOF | splash10-0f79-0009200000-0f92e092c978feb7ef4a | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 24-Hydroxycholesterol 20V, Positive-QTOF | splash10-00kr-4119100000-6a8569b9873ebded521a | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 24-Hydroxycholesterol 40V, Positive-QTOF | splash10-0c01-5049000000-3466fdb5c0a23399bea3 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 24-Hydroxycholesterol 10V, Negative-QTOF | splash10-0udi-0003900000-db2e1b276e6052150b6f | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 24-Hydroxycholesterol 20V, Negative-QTOF | splash10-0ue9-0009700000-55fe1a48489ad8aa8bb6 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 24-Hydroxycholesterol 40V, Negative-QTOF | splash10-00ri-7009000000-7a2a5d9fa1c30ba0c55b | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 24-Hydroxycholesterol 10V, Positive-QTOF | splash10-00ku-0209100000-bfd7a93b49439544b96f | 2021-09-23 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 24-Hydroxycholesterol 20V, Positive-QTOF | splash10-090u-5459000000-c9b30378df2d7c3e159c | 2021-09-23 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 24-Hydroxycholesterol 40V, Positive-QTOF | splash10-0a4l-6940000000-78cdb9ace90e3772c7c2 | 2021-09-23 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 24-Hydroxycholesterol 10V, Negative-QTOF | splash10-0udi-0000900000-079f76b4d985e4b92c9d | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 24-Hydroxycholesterol 20V, Negative-QTOF | splash10-0udi-0003900000-390ce6ee953a3d06105b | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 24-Hydroxycholesterol 40V, Negative-QTOF | splash10-0002-0009000000-b4881863f9f5f7cd2223 | 2021-09-24 | Wishart Lab | View Spectrum |
| Show more...
|---|
| Biological Properties |
|---|
| Cellular Locations | |
|---|
| Biospecimen Locations | - Blood
- Cerebrospinal Fluid (CSF)
- Feces
|
|---|
| Tissue Locations | Not Available |
|---|
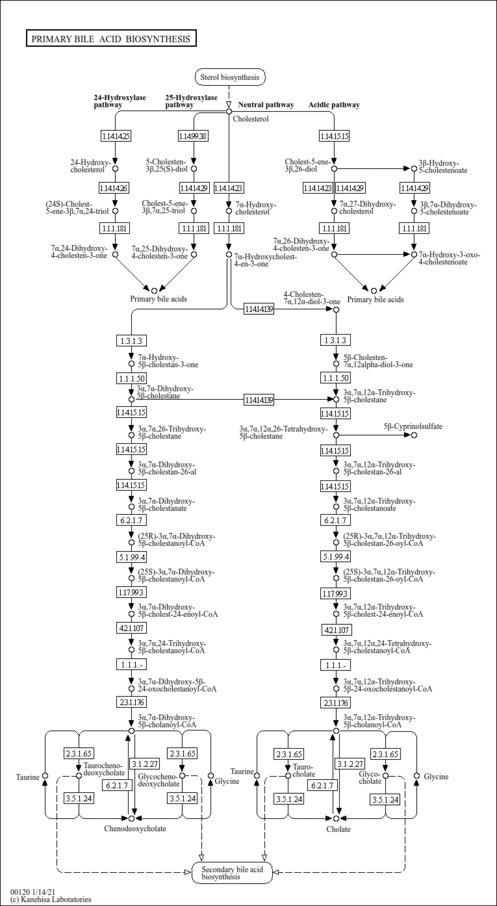
| Pathways | |
|---|
| Normal Concentrations |
|---|
| |
| Blood | Detected and Quantified | 0.049 +/- 0.002 uM | Adult (>18 years old) | Both | Normal | | details | | Blood | Detected and Quantified | 0.006 +/- 0.0003 uM | Adult (>18 years old) | Both | Normal | | details | | Blood | Detected and Quantified | 0.0018 +/- 0.00052 uM | Adult (>18 years old) | Both | Normal | | details | | Blood | Detected and Quantified | 0.191 +/- 0.00993 uM | Adult (>18 years old) | Both | Normal | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 0.0034 (0.0033-0.0036) uM | Adult (>18 years old) | Both | Normal | | details |
|
|---|
| Abnormal Concentrations |
|---|
| |
| Blood | Detected and Quantified | 0.11 +/- 0.03 uM | Adult (>18 years old) | Not Specified | Hypercholesterolemia | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 0.0061 (0.0058-0.0064) uM | Elderly (>65 years old) | Both | Alzheimer's disease | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 0.016 (0.014-0.017) uM | Adult (>18 years old) | Both | Acute or chronic demyelinating polyneuropathies | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 0.0045 (0.0041-0.0050) uM | Adult (>18 years old) | Both | Subarachnoid hemorrhage | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 0.0086 (0.0076-0.0096) uM | Adult (>18 years old) | Both | Meningitis | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 0.0062 (0.0057-0.0068) uM | Adult (>18 years old) | Both | Neuroborreliosis | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 0.0077 (0.0066-0.0093) uM | Adult (>18 years old) | Not Specified | Mild cognitive impairment | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 0.004 +/- 0.0013 uM | Adult (>18 years old) | Both | Multiple Sclerosis | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Inflammatory bowel disease | | details |
| Show more...
|---|
| Associated Disorders and Diseases |
|---|
| Disease References | | Hypercholesterolemia |
|---|
- Thelen KM, Laaksonen R, Paiva H, Lehtimaki T, Lutjohann D: High-dose statin treatment does not alter plasma marker for brain cholesterol metabolism in patients with moderately elevated plasma cholesterol levels. J Clin Pharmacol. 2006 Jul;46(7):812-6. [PubMed:16809807 ]
| | Alzheimer's disease |
|---|
- Leoni V, Masterman T, Mousavi FS, Wretlind B, Wahlund LO, Diczfalusy U, Hillert J, Bjorkhem I: Diagnostic use of cerebral and extracerebral oxysterols. Clin Chem Lab Med. 2004 Feb;42(2):186-91. [PubMed:15061359 ]
| | Demyelinating polyneuropathy |
|---|
- Leoni V, Masterman T, Mousavi FS, Wretlind B, Wahlund LO, Diczfalusy U, Hillert J, Bjorkhem I: Diagnostic use of cerebral and extracerebral oxysterols. Clin Chem Lab Med. 2004 Feb;42(2):186-91. [PubMed:15061359 ]
| | Subarachnoid hemorrhage |
|---|
- Leoni V, Masterman T, Mousavi FS, Wretlind B, Wahlund LO, Diczfalusy U, Hillert J, Bjorkhem I: Diagnostic use of cerebral and extracerebral oxysterols. Clin Chem Lab Med. 2004 Feb;42(2):186-91. [PubMed:15061359 ]
| | Meningitis |
|---|
- Leoni V, Masterman T, Mousavi FS, Wretlind B, Wahlund LO, Diczfalusy U, Hillert J, Bjorkhem I: Diagnostic use of cerebral and extracerebral oxysterols. Clin Chem Lab Med. 2004 Feb;42(2):186-91. [PubMed:15061359 ]
| | Neuroborreliosis |
|---|
- Leoni V, Masterman T, Mousavi FS, Wretlind B, Wahlund LO, Diczfalusy U, Hillert J, Bjorkhem I: Diagnostic use of cerebral and extracerebral oxysterols. Clin Chem Lab Med. 2004 Feb;42(2):186-91. [PubMed:15061359 ]
| | Mild cognitive impairment |
|---|
- Leoni V, Masterman T, Mousavi FS, Wretlind B, Wahlund LO, Diczfalusy U, Hillert J, Bjorkhem I: Diagnostic use of cerebral and extracerebral oxysterols. Clin Chem Lab Med. 2004 Feb;42(2):186-91. [PubMed:15061359 ]
| | Multiple sclerosis |
|---|
- Leoni V, Lutjohann D, Masterman T: Levels of 7-oxocholesterol in cerebrospinal fluid are more than one thousand times lower than reported in multiple sclerosis. J Lipid Res. 2005 Feb;46(2):191-5. Epub 2004 Dec 1. [PubMed:15576852 ]
| | Inflammatory bowel disease |
|---|
- Lee T, Clavel T, Smirnov K, Schmidt A, Lagkouvardos I, Walker A, Lucio M, Michalke B, Schmitt-Kopplin P, Fedorak R, Haller D: Oral versus intravenous iron replacement therapy distinctly alters the gut microbiota and metabolome in patients with IBD. Gut. 2017 May;66(5):863-871. doi: 10.1136/gutjnl-2015-309940. Epub 2016 Feb 4. [PubMed:26848182 ]
|
|
|---|
| Associated OMIM IDs | |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| Phenol Explorer Compound ID | Not Available |
|---|
| FooDB ID | FDB022611 |
|---|
| KNApSAcK ID | Not Available |
|---|
| Chemspider ID | 108790 |
|---|
| KEGG Compound ID | C13550 |
|---|
| BioCyc ID | Not Available |
|---|
| BiGG ID | 2197434 |
|---|
| Wikipedia Link | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PubChem Compound | 121948 |
|---|
| PDB ID | Not Available |
|---|
| ChEBI ID | 34310 |
|---|
| Food Biomarker Ontology | Not Available |
|---|
| VMH ID | XOL24OH |
|---|
| MarkerDB ID | MDB00000322 |
|---|
| Good Scents ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Prasad V V; Ponticorvo L; Lieberman S Identification of 24-hydroxycholesterol in bovine adrenals in both free and esterified forms and in bovine brains as its sulfate ester. Journal of steroid biochemistry (1984), 21(6), 733-6. |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| General References | - Leoni V, Masterman T, Mousavi FS, Wretlind B, Wahlund LO, Diczfalusy U, Hillert J, Bjorkhem I: Diagnostic use of cerebral and extracerebral oxysterols. Clin Chem Lab Med. 2004 Feb;42(2):186-91. [PubMed:15061359 ]
- Lutjohann D, von Bergmann K: 24S-hydroxycholesterol: a marker of brain cholesterol metabolism. Pharmacopsychiatry. 2003 Sep;36 Suppl 2:S102-6. [PubMed:14574622 ]
|
|---|