| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Expected but not Quantified |
|---|
| Creation Date | 2006-08-13 13:42:26 UTC |
|---|
| Update Date | 2023-02-21 17:16:57 UTC |
|---|
| HMDB ID | HMDB0004181 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Methylimidazole acetaldehyde |
|---|
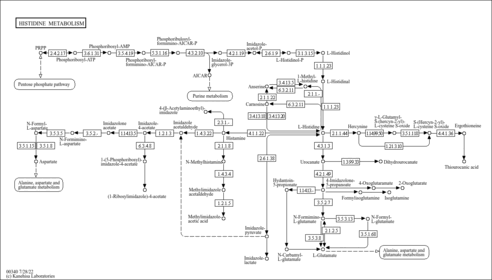
| Description | Methylimidazole acetaldehyde belongs to the class of organic compounds known as n-substituted imidazoles. These are heterocyclic compounds containing an imidazole ring substituted at position 1. Methylimidazole acetaldehyde is a very strong basic compound (based on its pKa). Methylimidazole acetaldehyde exists in all living organisms, ranging from bacteria to humans. Within humans, methylimidazole acetaldehyde participates in a number of enzymatic reactions. In particular, methylimidazole acetaldehyde can be biosynthesized from 1-methylhistamine through its interaction with the enzyme amine oxidase [flavin-containing] a. In addition, methylimidazole acetaldehyde can be converted into methylimidazoleacetic acid through the action of the enzyme aldehyde dehydrogenase, dimeric nadp-preferring. In humans, methylimidazole acetaldehyde is involved in histidine metabolism. |
|---|
| Structure | InChI=1S/C6H8N2O/c1-8-4-6(2-3-9)7-5-8/h3-5H,2H2,1H3 |
|---|
| Synonyms | | Value | Source |
|---|
| Methylimidazoleacetaldehyde | ChEBI | | 1-Methylimidazole-4-acetaldehyde | Kegg | | 1-Methylimidazol-3-ylacetaldehyde | HMDB | | N-Methylimidazole-3-acetaldehyde | HMDB |
|
|---|
| Chemical Formula | C6H8N2O |
|---|
| Average Molecular Weight | 124.1405 |
|---|
| Monoisotopic Molecular Weight | 124.063662888 |
|---|
| IUPAC Name | 2-(1-methyl-1H-imidazol-4-yl)acetaldehyde |
|---|
| Traditional Name | methylimidazole acetaldehyde |
|---|
| CAS Registry Number | 19639-03-3 |
|---|
| SMILES | CN1C=NC(CC=O)=C1 |
|---|
| InChI Identifier | InChI=1S/C6H8N2O/c1-8-4-6(2-3-9)7-5-8/h3-5H,2H2,1H3 |
|---|
| InChI Key | GCQHUBANENYTLB-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as n-substituted imidazoles. These are heterocyclic compounds containing an imidazole ring substituted at position 1. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Azoles |
|---|
| Sub Class | Imidazoles |
|---|
| Direct Parent | N-substituted imidazoles |
|---|
| Alternative Parents | |
|---|
| Substituents | - N-substituted imidazole
- Heteroaromatic compound
- Alpha-hydrogen aldehyde
- Azacycle
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Aldehyde
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Physiological effect | Not Available |
|---|
| Disposition | |
|---|
| Process | |
|---|
| Role | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | -0.067 | Not Available |
|
|---|
| Experimental Chromatographic Properties | Not Available |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Kovats Retention IndicesUnderivatizedDerivatized| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| Methylimidazole acetaldehyde,1TMS,isomer #1 | CN1C=NC(C=CO[Si](C)(C)C)=C1 | 1519.2 | Semi standard non polar | 33892256 | | Methylimidazole acetaldehyde,1TMS,isomer #1 | CN1C=NC(C=CO[Si](C)(C)C)=C1 | 1586.0 | Standard non polar | 33892256 | | Methylimidazole acetaldehyde,1TMS,isomer #1 | CN1C=NC(C=CO[Si](C)(C)C)=C1 | 1852.6 | Standard polar | 33892256 | | Methylimidazole acetaldehyde,1TBDMS,isomer #1 | CN1C=NC(C=CO[Si](C)(C)C(C)(C)C)=C1 | 1764.7 | Semi standard non polar | 33892256 | | Methylimidazole acetaldehyde,1TBDMS,isomer #1 | CN1C=NC(C=CO[Si](C)(C)C(C)(C)C)=C1 | 1785.2 | Standard non polar | 33892256 | | Methylimidazole acetaldehyde,1TBDMS,isomer #1 | CN1C=NC(C=CO[Si](C)(C)C(C)(C)C)=C1 | 2032.0 | Standard polar | 33892256 |
|
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - Methylimidazole acetaldehyde GC-MS (Non-derivatized) - 70eV, Positive | splash10-00ed-9300000000-e28295e6eb9bf8f2b88f | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Methylimidazole acetaldehyde GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Methylimidazole acetaldehyde GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Methylimidazole acetaldehyde 10V, Positive-QTOF | splash10-004i-0900000000-5f767a0fec7621be3a2c | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Methylimidazole acetaldehyde 20V, Positive-QTOF | splash10-056r-2900000000-13f4edfe17ee48d567ea | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Methylimidazole acetaldehyde 40V, Positive-QTOF | splash10-1000-9000000000-a759eba091ef0dedb4b4 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Methylimidazole acetaldehyde 10V, Negative-QTOF | splash10-00di-1900000000-55dc09aa08cf80ab1c31 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Methylimidazole acetaldehyde 20V, Negative-QTOF | splash10-00dj-5900000000-dba8549393f4f86d9eb8 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Methylimidazole acetaldehyde 40V, Negative-QTOF | splash10-0a4l-9100000000-033ab68de508c9aab9fa | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Methylimidazole acetaldehyde 10V, Positive-QTOF | splash10-003s-9700000000-8c686087fce0e37ab3e0 | 2021-09-23 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Methylimidazole acetaldehyde 20V, Positive-QTOF | splash10-0k92-9200000000-aa26a9fad148965cd8bd | 2021-09-23 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Methylimidazole acetaldehyde 40V, Positive-QTOF | splash10-00l6-9000000000-e94b9bbeddb75c9885ff | 2021-09-23 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Methylimidazole acetaldehyde 10V, Negative-QTOF | splash10-00di-2900000000-88175458595aff08ad02 | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Methylimidazole acetaldehyde 20V, Negative-QTOF | splash10-00ec-9400000000-8e831092ddfad5f29abd | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Methylimidazole acetaldehyde 40V, Negative-QTOF | splash10-00kf-9000000000-169c6ba262d1f7d5a362 | 2021-09-24 | Wishart Lab | View Spectrum |
NMR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum |
IR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M+H]+) | 2023-02-03 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M+Na]+) | 2023-02-03 | FELIX lab | View Spectrum |
|
|---|