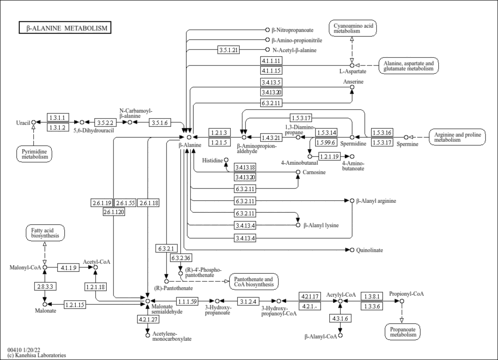
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - Anserine GC-MS (Non-derivatized) - 70eV, Positive | splash10-008a-9110000000-2f66d9e2bd58d8fdfe22 | 2016-09-22 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Anserine GC-MS (1 TMS) - 70eV, Positive | splash10-008a-9220000000-c098b7478e4e2469402c | 2017-10-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Anserine GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Anserine GC-MS (TMS_1_2) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Anserine GC-MS (TMS_1_3) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Anserine GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Anserine GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Anserine GC-MS (TBDMS_1_3) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental LC-MS/MS | LC-MS/MS Spectrum - Anserine Quattro_QQQ 10V, Positive-QTOF (Annotated) | splash10-0a4i-0910000000-e359d14a1164b571973e | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Anserine Quattro_QQQ 25V, Positive-QTOF (Annotated) | splash10-0a4i-0900000000-3ae24b5b69cb5463a956 | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Anserine Quattro_QQQ 40V, Positive-QTOF (Annotated) | splash10-0002-7930000000-344e4a7f3b532ce31015 | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Anserine LC-ESI-QQ (API3000, Applied Biosystems) 10V, Negative-QTOF | splash10-000i-0090000000-66959f5b2f8cd0fed35d | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Anserine LC-ESI-QQ (API3000, Applied Biosystems) 20V, Negative-QTOF | splash10-014r-0950000000-27db9dc4c4fbe3889f7d | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Anserine LC-ESI-QQ (API3000, Applied Biosystems) 30V, Negative-QTOF | splash10-014i-3900000000-e8dd832f2b9453b196fb | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Anserine LC-ESI-QQ (API3000, Applied Biosystems) 40V, Negative-QTOF | splash10-0a4i-9500000000-91673df79b5fb9e1373b | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Anserine LC-ESI-QQ (API3000, Applied Biosystems) 50V, Negative-QTOF | splash10-0a4i-9100000000-468135f3bc158f013255 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Anserine LC-ESI-QQ (API3000, Applied Biosystems) 10V, Positive-QTOF | splash10-0006-0090000000-5545eb5f0143bc37b54c | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Anserine LC-ESI-QQ (API3000, Applied Biosystems) 20V, Positive-QTOF | splash10-0006-0690000000-65defef60d9fd6e60fe8 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Anserine LC-ESI-QQ (API3000, Applied Biosystems) 30V, Positive-QTOF | splash10-0a4i-1900000000-1ecb5ca2c9f4935af629 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Anserine LC-ESI-QQ (API3000, Applied Biosystems) 40V, Positive-QTOF | splash10-0a4j-5900000000-24168da8606d1994f23b | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Anserine LC-ESI-QQ (API3000, Applied Biosystems) 50V, Positive-QTOF | splash10-052b-9400000000-08a9a8a8d83e5958d60e | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Anserine LC-ESI-IT (LC/MSD Trap XCT, Agilent Technologies) , Positive-QTOF | splash10-00di-0920000000-fdb3cabe02ef9f20956d | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Anserine LC-ESI-IT (LC/MSD Trap XCT, Agilent Technologies) , Positive-QTOF | splash10-0a4i-0900000000-39c6787170775cf28b30 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Anserine LC-ESI-IT (LC/MSD Trap XCT, Agilent Technologies) , Positive-QTOF | splash10-0a4i-1900000000-a9ee2ab9908f584909b4 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Anserine LC-ESI-IT (LC/MSD Trap XCT, Agilent Technologies) , Positive-QTOF | splash10-0aor-6900000000-934d365646fde55d5c16 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Anserine LC-ESI-IT (LC/MSD Trap XCT, Agilent Technologies) , Positive-QTOF | splash10-0a4i-0900000000-440a3e57f35809ac4568 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Anserine LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Positive-QTOF | splash10-052g-3950000000-656f64d2f4533d03073e | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Anserine LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Negative-QTOF | splash10-014r-0960000000-1ec10daf4624249b1d5b | 2012-08-31 | HMDB team, MONA | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Anserine 10V, Positive-QTOF | splash10-00xu-1970000000-1e25ebc6c091a98fd2e9 | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Anserine 20V, Positive-QTOF | splash10-00di-3910000000-731cfc3c9901fad1d44b | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Anserine 40V, Positive-QTOF | splash10-00di-3900000000-307d922cb29b8694f151 | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Anserine 10V, Negative-QTOF | splash10-000i-1490000000-b93b05b7108f994cf9f6 | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Anserine 20V, Negative-QTOF | splash10-0079-5950000000-42d4878fd04fd2f8210e | 2016-09-12 | Wishart Lab | View Spectrum |
NMR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted 1D NMR | 13C NMR Spectrum (1D, 100 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 100 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 200 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 200 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 300 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 300 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 400 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 400 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 500 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 500 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 600 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 700 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 700 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 800 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 800 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 900 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 900 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Experimental 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, H2O, experimental) | 2012-12-05 | Wishart Lab | View Spectrum |
| Show more...
|---|
| General References | - Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, Laxman B, Mehra R, Lonigro RJ, Li Y, Nyati MK, Ahsan A, Kalyana-Sundaram S, Han B, Cao X, Byun J, Omenn GS, Ghosh D, Pennathur S, Alexander DC, Berger A, Shuster JR, Wei JT, Varambally S, Beecher C, Chinnaiyan AM: Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009 Feb 12;457(7231):910-4. doi: 10.1038/nature07762. [PubMed:19212411 ]
- Harris RC, Tallon MJ, Dunnett M, Boobis L, Coakley J, Kim HJ, Fallowfield JL, Hill CA, Sale C, Wise JA: The absorption of orally supplied beta-alanine and its effect on muscle carnosine synthesis in human vastus lateralis. Amino Acids. 2006 May;30(3):279-89. Epub 2006 Mar 24. [PubMed:16554972 ]
- Pagnano MW, Hanssen AD, Lewallen DG, Stuart MJ: Flexion instability after primary posterior cruciate retaining total knee arthroplasty. Clin Orthop Relat Res. 1998 Nov;(356):39-46. [PubMed:9917666 ]
- Abe H, Okuma E, Sekine H, Maeda A, Yoshiue S: Human urinary excretion of L-histidine-related compounds after ingestion of several meats and fish muscle. Int J Biochem. 1993 Sep;25(9):1245-9. [PubMed:8224369 ]
- Kang I, Han SW: Anserine bursitis in patients with osteoarthritis of the knee. South Med J. 2000 Feb;93(2):207-9. [PubMed:10701790 ]
- Magana Loarte JE, Perez Franco J, Sanchez Sanchez G: [Is therapy with local infiltrations feasible in primary care consultations?]. Aten Primaria. 1999 Jan;23(1):4-7. [PubMed:10079554 ]
- Tan KM, Candlish JK: Carnosine and anserine as modulators of neutrophil function. Clin Lab Haematol. 1998 Aug;20(4):239-44. [PubMed:9777271 ]
- Boldyrev A, Abe H: Metabolic transformation of neuropeptide carnosine modifies its biological activity. Cell Mol Neurobiol. 1999 Feb;19(1):163-75. [PubMed:10079975 ]
- Elshenawy S, Pinney SE, Stuart T, Doulias PT, Zura G, Parry S, Elovitz MA, Bennett MJ, Bansal A, Strauss JF 3rd, Ischiropoulos H, Simmons RA: The Metabolomic Signature of the Placenta in Spontaneous Preterm Birth. Int J Mol Sci. 2020 Feb 4;21(3). pii: ijms21031043. doi: 10.3390/ijms21031043. [PubMed:32033212 ]
- Kwiatkowski S, Kiersztan A, Drozak J: Biosynthesis of Carnosine and Related Dipeptides in Vertebrates. Curr Protein Pept Sci. 2018;19(8):771-789. doi: 10.2174/1389203719666180226155657. [PubMed:29484990 ]
- Drozak J, Chrobok L, Poleszak O, Jagielski AK, Derlacz R: Molecular identification of carnosine N-methyltransferase as chicken histamine N-methyltransferase-like protein (hnmt-like). PLoS One. 2013 May 21;8(5):e64805. doi: 10.1371/journal.pone.0064805. Print 2013. [PubMed:23705015 ]
| Show more...
|---|