| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Detected and Quantified |
|---|
| Creation Date | 2005-11-16 15:48:42 UTC |
|---|
| Update Date | 2021-09-14 14:57:20 UTC |
|---|
| HMDB ID | HMDB0000902 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | NAD |
|---|
| Description | NAD (or nicotinamide adenine dinucleotide) is used extensively in glycolysis and the citric acid cycle of cellular respiration. The reducing potential stored in NADH can be either converted into ATP through the electron transport chain or used for anabolic metabolism. ATP "energy" is necessary for an organism to live. Green plants obtain ATP through photosynthesis, while other organisms obtain it via cellular respiration (Wikipedia ). NAD is a coenzyme composed of ribosylnicotinamide 5'-diphosphate coupled to adenosine 5'-phosphate by a pyrophosphate linkage. It is found widely in nature and is involved in numerous enzymatic reactions in which it serves as an electron carrier by being alternately oxidized (NAD+) and reduced (NADH) (Dorland, 27th ed). |
|---|
| Structure | NC(=O)C1=C[N+](=CC=C1)[C@@H]1O[C@H](COP([O-])(=O)OP(O)(=O)OC[C@H]2O[C@H]([C@H](O)[C@@H]2O)N2C=NC3=C2N=CN=C3N)[C@@H](O)[C@H]1O InChI=1S/C21H27N7O14P2/c22-17-12-19(25-7-24-17)28(8-26-12)21-16(32)14(30)11(41-21)6-39-44(36,37)42-43(34,35)38-5-10-13(29)15(31)20(40-10)27-3-1-2-9(4-27)18(23)33/h1-4,7-8,10-11,13-16,20-21,29-32H,5-6H2,(H5-,22,23,24,25,33,34,35,36,37)/t10-,11-,13-,14-,15-,16-,20-,21-/m1/s1 |
|---|
| Synonyms | | Value | Source |
|---|
| Codehydrogenase I | ChEBI | | Coenzyme I | ChEBI | | Cozymase I | ChEBI | | Diphosphopyridine nucleotide | ChEBI | | DPN | ChEBI | | Nadide | ChEBI | | NICOTINAMIDE-adenine-dinucleotide | ChEBI | | Adenine dinucleotide, dihydronicotinamide | MeSH | | NAD | MeSH | | Nicotinamide-adenine dinucleotide | MeSH | | Nucleotide, diphosphopyridine | MeSH | | Dihydronicotinamide adenine dinucleotide | MeSH | | NADH | MeSH | | Nicotinamide adenine dinucleotide | MeSH | | Dinucleotide, nicotinamide-adenine | MeSH | | Dinucleotide, dihydronicotinamide adenine | MeSH | | Adenine-nicotinamide dinucleotide | HMDB | | NAD+ | HMDB | | Oxidized diphosphopyridine nucleotide | HMDB | | beta-Diphosphopyridine nucleotide | HMDB | | beta-NAD | HMDB | | beta-NAD+ | HMDB | | beta-Nicotinamide adenine dinucleotide | HMDB | | beta-Nicotinamide adenine dinucleotide hydrate | HMDB | | β-Diphosphopyridine nucleotide | HMDB | | β-NAD | HMDB | | β-NAD+ | HMDB | | β-Nicotinamide adenine dinucleotide | HMDB | | β-Nicotinamide adenine dinucleotide hydrate | HMDB |
|
|---|
| Chemical Formula | C21H27N7O14P2 |
|---|
| Average Molecular Weight | 663.4251 |
|---|
| Monoisotopic Molecular Weight | 663.109121631 |
|---|
| IUPAC Name | 1-[(2R,3R,4S,5R)-5-({[({[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxyoxolan-2-yl]methyl phosphono}oxy)(hydroxy)phosphoryl]oxy}methyl)-3,4-dihydroxyoxolan-2-yl]-3-(C-hydroxycarbonimidoyl)-1lambda5-pyridin-1-ylium |
|---|
| Traditional Name | 1-[(2R,3R,4S,5R)-5-{[({[(2R,3S,4R,5R)-5-(6-aminopurin-9-yl)-3,4-dihydroxyoxolan-2-yl]methyl phosphono}oxy(hydroxy)phosphoryl)oxy]methyl}-3,4-dihydroxyoxolan-2-yl]-3-(C-hydroxycarbonimidoyl)-1lambda5-pyridin-1-ylium |
|---|
| CAS Registry Number | 53-84-9 |
|---|
| SMILES | NC(=O)C1=C[N+](=CC=C1)[C@@H]1O[C@H](COP([O-])(=O)OP(O)(=O)OC[C@H]2O[C@H]([C@H](O)[C@@H]2O)N2C=NC3=C2N=CN=C3N)[C@@H](O)[C@H]1O |
|---|
| InChI Identifier | InChI=1S/C21H27N7O14P2/c22-17-12-19(25-7-24-17)28(8-26-12)21-16(32)14(30)11(41-21)6-39-44(36,37)42-43(34,35)38-5-10-13(29)15(31)20(40-10)27-3-1-2-9(4-27)18(23)33/h1-4,7-8,10-11,13-16,20-21,29-32H,5-6H2,(H5-,22,23,24,25,33,34,35,36,37)/t10-,11-,13-,14-,15-,16-,20-,21-/m1/s1 |
|---|
| InChI Key | BAWFJGJZGIEFAR-NNYOXOHSSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as (5'->5')-dinucleotides. These are dinucleotides where the two bases are connected via a (5'->5')-phosphodiester linkage. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Nucleosides, nucleotides, and analogues |
|---|
| Class | (5'->5')-dinucleotides |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | (5'->5')-dinucleotides |
|---|
| Alternative Parents | |
|---|
| Substituents | - (5'->5')-dinucleotide
- Purine nucleotide sugar
- Purine ribonucleoside diphosphate
- Purine ribonucleoside monophosphate
- Nicotinamide-nucleotide
- Pyridine nucleotide
- Pentose-5-phosphate
- Pentose phosphate
- Glycosyl compound
- N-glycosyl compound
- 6-aminopurine
- Monosaccharide phosphate
- Organic pyrophosphate
- Purine
- Imidazopyrimidine
- Nicotinamide
- Monoalkyl phosphate
- Aminopyrimidine
- Monosaccharide
- N-substituted imidazole
- Organic phosphoric acid derivative
- Imidolactam
- Phosphoric acid ester
- Alkyl phosphate
- Pyrimidine
- Pyridinium
- Pyridine
- Tetrahydrofuran
- Azole
- Imidazole
- Heteroaromatic compound
- Secondary alcohol
- Carboximidic acid derivative
- Carboximidic acid
- Organoheterocyclic compound
- Oxacycle
- Azacycle
- Hydrocarbon derivative
- Organopnictogen compound
- Organic oxide
- Organic zwitterion
- Primary amine
- Organonitrogen compound
- Amine
- Organic nitrogen compound
- Alcohol
- Organic oxygen compound
- Organooxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Not Available | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | 140.0 - 142.0 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 752.5 mg/mL | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Experimental Collision Cross Sections| Adduct Type | Data Source | CCS Value (Å2) | Reference |
|---|
| [M-H]- | Astarita_neg | 226.3 | 30932474 |
|
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Retention Times Underivatized| Chromatographic Method | Retention Time | Reference |
|---|
| Measured using a Waters Acquity ultraperformance liquid chromatography (UPLC) ethylene-bridged hybrid (BEH) C18 column (100 mm × 2.1 mm; 1.7 μmparticle diameter). Predicted by Afia on May 17, 2022. Predicted by Afia on May 17, 2022. | 2.55 minutes | 32390414 | | Predicted by Siyang on May 30, 2022 | 9.9879 minutes | 33406817 | | Predicted by Siyang using ReTip algorithm on June 8, 2022 | 9.2 minutes | 32390414 | | AjsUoB = Accucore 150 Amide HILIC with 10mM Ammonium Formate, 0.1% Formic Acid | 448.8 seconds | 40023050 | | Fem_Long = Waters ACQUITY UPLC HSS T3 C18 with Water:MeOH and 0.1% Formic Acid | 1141.7 seconds | 40023050 | | Fem_Lipids = Ascentis Express C18 with (60:40 water:ACN):(90:10 IPA:ACN) and 10mM NH4COOH + 0.1% Formic Acid | 187.4 seconds | 40023050 | | Life_Old = Waters ACQUITY UPLC BEH C18 with Water:(20:80 acetone:ACN) and 0.1% Formic Acid | 67.2 seconds | 40023050 | | Life_New = RP Waters ACQUITY UPLC HSS T3 C18 with Water:(30:70 MeOH:ACN) and 0.1% Formic Acid | 157.5 seconds | 40023050 | | RIKEN = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 62.1 seconds | 40023050 | | Eawag_XBridgeC18 = XBridge C18 3.5u 2.1x50 mm with Water:MeOH and 0.1% Formic Acid | 344.6 seconds | 40023050 | | BfG_NTS_RP1 =Agilent Zorbax Eclipse Plus C18 (2.1 mm x 150 mm, 3.5 um) with Water:ACN and 0.1% Formic Acid | 349.5 seconds | 40023050 | | HILIC_BDD_2 = Merck SeQuant ZIC-HILIC with ACN(0.1% formic acid):water(16 mM ammonium formate) | 894.4 seconds | 40023050 | | UniToyama_Atlantis = RP Waters Atlantis T3 (2.1 x 150 mm, 5 um) with ACN:Water and 0.1% Formic Acid | 624.3 seconds | 40023050 | | BDD_C18 = Hypersil Gold 1.9µm C18 with Water:ACN and 0.1% Formic Acid | 116.5 seconds | 40023050 | | UFZ_Phenomenex = Kinetex Core-Shell C18 2.6 um, 3.0 x 100 mm, Phenomenex with Water:MeOH and 0.1% Formic Acid | 779.3 seconds | 40023050 | | SNU_RIKEN_POS = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 197.1 seconds | 40023050 | | RPMMFDA = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 236.8 seconds | 40023050 | | MTBLS87 = Merck SeQuant ZIC-pHILIC column with ACN:Water and :ammonium carbonate | 502.4 seconds | 40023050 | | KI_GIAR_zic_HILIC_pH2_7 = Merck SeQuant ZIC-HILIC with ACN:Water and 0.1% FA | 382.8 seconds | 40023050 | | Meister zic-pHILIC pH9.3 = Merck SeQuant ZIC-pHILIC column with ACN:Water 5mM NH4Ac pH9.3 and 5mM ammonium acetate in water | 442.6 seconds | 40023050 |
Predicted Kovats Retention IndicesUnderivatized |
|---|
| Spectra |
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - NAD GC-MS (TMS_1_1) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NAD GC-MS (TMS_1_2) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NAD GC-MS (TMS_1_3) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NAD GC-MS (TMS_1_4) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NAD GC-MS (TMS_1_5) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NAD GC-MS (TMS_1_6) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NAD GC-MS (TMS_1_7) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NAD GC-MS (TMS_2_1) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NAD GC-MS (TMS_2_2) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NAD GC-MS (TMS_2_3) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NAD GC-MS (TMS_2_4) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NAD GC-MS (TMS_2_5) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NAD GC-MS (TMS_2_6) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NAD GC-MS (TMS_2_7) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NAD GC-MS (TMS_2_8) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NAD GC-MS (TMS_2_9) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NAD GC-MS (TMS_2_10) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NAD GC-MS (TMS_2_11) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NAD GC-MS (TMS_2_12) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NAD GC-MS (TMS_2_13) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NAD GC-MS (TMS_2_14) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NAD GC-MS (TMS_2_15) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NAD GC-MS (TMS_2_16) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NAD GC-MS (TMS_2_17) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NAD GC-MS (TMS_2_18) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental LC-MS/MS | LC-MS/MS Spectrum - NAD Quattro_QQQ 10V, Positive-QTOF (Annotated) | splash10-03di-0000029000-870aa620464a4fedbe8d | 2019-11-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - NAD Quattro_QQQ 25V, Positive-QTOF (Annotated) | splash10-002r-0930610000-ccd233f26036136ba3e8 | 2019-11-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - NAD Quattro_QQQ 40V, Positive-QTOF (Annotated) | splash10-000i-0900000000-ec77ba41ae7dbcfd08a2 | 2019-11-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - NAD LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive-QTOF | splash10-00dl-0219003700-2f52e3c5db41066a112c | 2019-11-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - NAD LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive-QTOF | splash10-0uk9-0301009000-a3d0c464e56f6e320c80 | 2019-11-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - NAD LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive-QTOF | splash10-00dl-0000090000-c18a7719161c63a71938 | 2019-11-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - NAD LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive-QTOF | splash10-0udi-0000009000-1dde5b221786fe375304 | 2019-11-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - NAD LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive-QTOF | splash10-0002-0911001000-bd16ca8021ab63e9e290 | 2019-11-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - NAD LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive-QTOF | splash10-001i-0900000000-662adda5a00fce5c5017 | 2019-11-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - NAD LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive-QTOF | splash10-03di-0900000000-a2724dbab2ca6eb7e8da | 2019-11-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - NAD LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive-QTOF | splash10-00dl-0400090000-19bb49ef6fe960f224d2 | 2019-11-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - NAD LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative-QTOF | splash10-006x-0011297400-efee4fe3a4cf024c960a | 2019-11-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - NAD LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative-QTOF | splash10-004i-0028900000-8ec9bcaf25513495b979 | 2019-11-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - NAD LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative-QTOF | splash10-0a4i-0011953000-577487fb6aff29c77330 | 2019-11-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - NAD LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative-QTOF | splash10-00di-0000009000-e1d0afb4e7926a0f845e | 2019-11-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - NAD LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative-QTOF | splash10-0006-0001092010-1dcbca7a5ffe61f23e50 | 2019-11-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - NAD LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative-QTOF | splash10-004i-0028900000-764e3ecea72fe178a87e | 2019-11-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - NAD LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative-QTOF | splash10-004i-0018900000-3b774fba9b96129baa6c | 2019-11-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - NAD LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative-QTOF | splash10-0006-0000090000-d4236efb9eec36017416 | 2019-11-22 | HMDB team, MONA | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - NAD 10V, Positive-QTOF | splash10-03di-0000009000-3cb1cc8e613a3e179149 | 2016-08-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - NAD 20V, Positive-QTOF | splash10-014j-0000009000-4bd400c0be81ab938465 | 2016-08-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - NAD 40V, Positive-QTOF | splash10-0006-6920102000-5fc300f474c5dc2b8600 | 2016-08-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - NAD 10V, Negative-QTOF | splash10-03di-0000009000-96d7faa48cdd181798a0 | 2016-08-03 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - NAD 20V, Negative-QTOF | splash10-03di-1100109000-f496601a0ac75ae3d4a5 | 2016-08-03 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - NAD 40V, Negative-QTOF | splash10-003s-9201000000-c06274c9b4d1b70a9ce2 | 2016-08-03 | Wishart Lab | View Spectrum |
NMR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Experimental 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, H2O, experimental) | 2019-11-22 | Wishart Lab | View Spectrum |
|
|---|
| Biological Properties |
|---|
| Cellular Locations | - Extracellular
- Mitochondria
- Nucleus
- Endoplasmic reticulum
- Peroxisome
|
|---|
| Biospecimen Locations | |
|---|
| Tissue Locations | - Adrenal Gland
- Brain
- Epidermis
- Fibroblasts
- Liver
- Placenta
- Platelet
- Prostate
- Skeletal Muscle
|
|---|
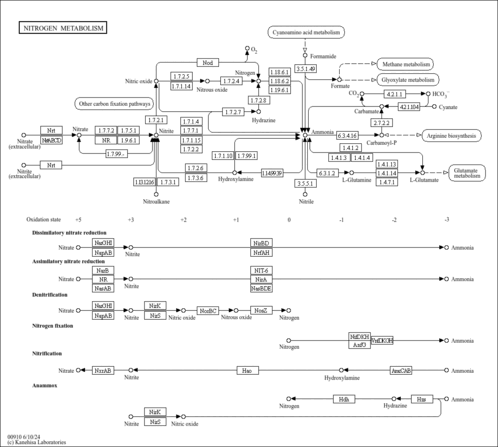
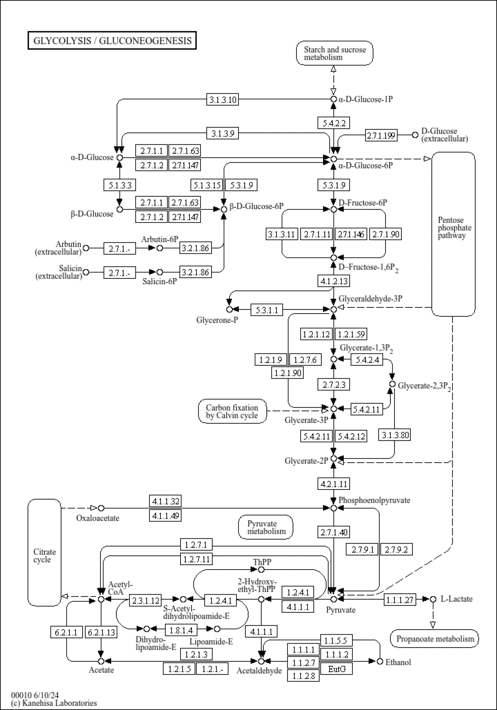
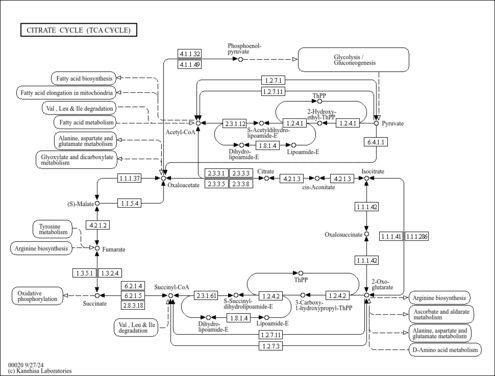
| Pathways | |
|---|
| Normal Concentrations |
|---|
| |
| Blood | Detected and Quantified | 24.3 (23.0-25.6) uM | Adult (>18 years old) | Both | Normal | | details | | Cellular Cytoplasm | Detected and Quantified | 88.7 uM | Adult (>18 years old) | Both | Normal | | details |
|
|---|
| Abnormal Concentrations |
|---|
| |
| Blood | Detected and Quantified | 29.1 (25.0-33.2) uM | Adult (>18 years old) | Both | Pellagra | | details | | Blood | Detected and Quantified | 0.0036 uM | Infant (0-1 year old) | Female | Nicotinamide Adenine Dinucleotide Deficiency | | details |
|
|---|
| Associated Disorders and Diseases |
|---|
| Disease References | | Pellagra |
|---|
- Creeke PI, Dibari F, Cheung E, van den Briel T, Kyroussis E, Seal AJ: Whole blood NAD and NADP concentrations are not depressed in subjects with clinical pellagra. J Nutr. 2007 Sep;137(9):2013-7. [PubMed:17709435 ]
| | Nicotinamide Adenine Dinucleotide Deficiency |
|---|
- Shi H, Enriquez A, Rapadas M, Martin EMMA, Wang R, Moreau J, Lim CK, Szot JO, Ip E, Hughes JN, Sugimoto K, Humphreys DT, McInerney-Leo AM, Leo PJ, Maghzal GJ, Halliday J, Smith J, Colley A, Mark PR, Collins F, Sillence DO, Winlaw DS, Ho JWK, Guillemin GJ, Brown MA, Kikuchi K, Thomas PQ, Stocker R, Giannoulatou E, Chapman G, Duncan EL, Sparrow DB, Dunwoodie SL: NAD Deficiency, Congenital Malformations, and Niacin Supplementation. N Engl J Med. 2017 Aug 10;377(6):544-552. doi: 10.1056/NEJMoa1616361. [PubMed:28792876 ]
|
|
|---|
| Associated OMIM IDs | None |
|---|
| External Links |
|---|
| DrugBank ID | DB14128 |
|---|
| Phenol Explorer Compound ID | Not Available |
|---|
| FooDB ID | FDB022309 |
|---|
| KNApSAcK ID | C00007256 |
|---|
| Chemspider ID | 5682 |
|---|
| KEGG Compound ID | C00003 |
|---|
| BioCyc ID | NAD |
|---|
| BiGG ID | Not Available |
|---|
| Wikipedia Link | Nicotinamide_adenine_dinucleotide |
|---|
| METLIN ID | Not Available |
|---|
| PubChem Compound | 5892 |
|---|
| PDB ID | Not Available |
|---|
| ChEBI ID | 44215 |
|---|
| Food Biomarker Ontology | Not Available |
|---|
| VMH ID | Not Available |
|---|
| MarkerDB ID | Not Available |
|---|
| Good Scents ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Hughes, N. A.; Kenner, G. W.; Todd, Alexander. Codehydrogenases. III. Synthesis of diphosphopyridine nucleotide (cozymase) and triphosphopyridine nucleotide. Journal of the Chemical Society (1957), 3733-8. |
|---|
| Material Safety Data Sheet (MSDS) | Download (PDF) |
|---|
| General References | - Brautigam CA, Chuang JL, Tomchick DR, Machius M, Chuang DT: Crystal structure of human dihydrolipoamide dehydrogenase: NAD+/NADH binding and the structural basis of disease-causing mutations. J Mol Biol. 2005 Jul 15;350(3):543-52. [PubMed:15946682 ]
- Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, Laxman B, Mehra R, Lonigro RJ, Li Y, Nyati MK, Ahsan A, Kalyana-Sundaram S, Han B, Cao X, Byun J, Omenn GS, Ghosh D, Pennathur S, Alexander DC, Berger A, Shuster JR, Wei JT, Varambally S, Beecher C, Chinnaiyan AM: Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009 Feb 12;457(7231):910-4. doi: 10.1038/nature07762. [PubMed:19212411 ]
- Nakayama Y, Kinoshita A, Tomita M: Dynamic simulation of red blood cell metabolism and its application to the analysis of a pathological condition. Theor Biol Med Model. 2005 May 9;2:18. [PubMed:15882454 ]
- Ying W: NAD+ and NADH in cellular functions and cell death. Front Biosci. 2006 Sep 1;11:3129-48. [PubMed:16720381 ]
- Hamza A, Cho H, Tai HH, Zhan CG: Understanding human 15-hydroxyprostaglandin dehydrogenase binding with NAD+ and PGE2 by homology modeling, docking and molecular dynamics simulation. Bioorg Med Chem. 2005 Jul 15;13(14):4544-51. [PubMed:15908215 ]
- Begonja AJ, Gambaryan S, Geiger J, Aktas B, Pozgajova M, Nieswandt B, Walter U: Platelet NAD(P)H-oxidase-generated ROS production regulates alphaIIbbeta3-integrin activation independent of the NO/cGMP pathway. Blood. 2005 Oct 15;106(8):2757-60. Epub 2005 Jun 23. [PubMed:15976180 ]
- Bruzzone S, Moreschi I, Guida L, Usai C, Zocchi E, De Flora A: Extracellular NAD+ regulates intracellular calcium levels and induces activation of human granulocytes. Biochem J. 2006 Feb 1;393(Pt 3):697-704. [PubMed:16225456 ]
- Kim MY, Zhang T, Kraus WL: Poly(ADP-ribosyl)ation by PARP-1: 'PAR-laying' NAD+ into a nuclear signal. Genes Dev. 2005 Sep 1;19(17):1951-67. [PubMed:16140981 ]
- Kunsman GW, Manno JE, Cockerham KR, Manno BR: A modification and validation of two urine ethanol procedures for use with the Monarch 2000 Chemistry System. J Anal Toxicol. 1991 May-Jun;15(3):130-5. [PubMed:1943056 ]
- Orczyk J, Morre DM, Morre DJ: Periodic fluctuations in oxygen consumption comparing HeLa (cancer) and CHO (non-cancer) cells and response to external NAD(P)+/NAD(P)H. Mol Cell Biochem. 2005 May;273(1-2):161-7. [PubMed:16013451 ]
- Krotz F, Sohn HY, Gloe T, Zahler S, Riexinger T, Schiele TM, Becker BF, Theisen K, Klauss V, Pohl U: NAD(P)H oxidase-dependent platelet superoxide anion release increases platelet recruitment. Blood. 2002 Aug 1;100(3):917-24. [PubMed:12130503 ]
- Elshenawy S, Pinney SE, Stuart T, Doulias PT, Zura G, Parry S, Elovitz MA, Bennett MJ, Bansal A, Strauss JF 3rd, Ischiropoulos H, Simmons RA: The Metabolomic Signature of the Placenta in Spontaneous Preterm Birth. Int J Mol Sci. 2020 Feb 4;21(3). pii: ijms21031043. doi: 10.3390/ijms21031043. [PubMed:32033212 ]
|
|---|