| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Expected but not Quantified |
|---|
| Creation Date | 2005-11-16 15:48:42 UTC |
|---|
| Update Date | 2023-02-21 17:15:23 UTC |
|---|
| HMDB ID | HMDB0001014 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 4-Imidazolone-5-propionic acid |
|---|
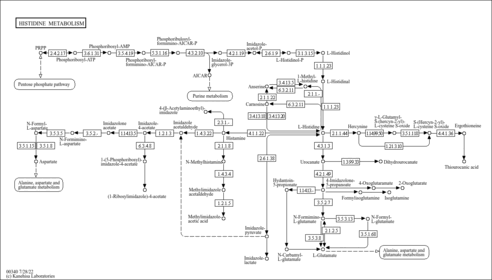
| Description | 4-Imidazolone-5-propionic acid, also known as 4-imidazolone-5-propanoate, belongs to the class of organic compounds known as imidazolyl carboxylic acids and derivatives. These are organic compounds containing a carboxylic acid chain (of at least 2 carbon atoms) linked to an imidazole ring. An imidazol-4-one having a 2-carboxyethyl substituent at the 5-position. 4-Imidazolone-5-propionic acid is a strong basic compound (based on its pKa). 4-Imidazolone-5-propionic acid exists in all living organisms, ranging from bacteria to humans. Within humans, 4-imidazolone-5-propionic acid participates in a number of enzymatic reactions. In particular, 4-imidazolone-5-propionic acid can be converted into urocanic acid through its interaction with the enzyme urocanate hydratase. In addition, 4-imidazolone-5-propionic acid can be converted into formiminoglutamic acid through the action of the enzyme probable imidazolonepropionase. In humans, 4-imidazolone-5-propionic acid is involved in histidine metabolism. |
|---|
| Structure | InChI=1S/C6H8N2O3/c9-5(10)2-1-4-6(11)8-3-7-4/h3-4H,1-2H2,(H,9,10)(H,7,8,11) |
|---|
| Synonyms | | Value | Source |
|---|
| 4,5-Dihydro-4-oxo-5-imidazolepropanoate | ChEBI | | 4,5-Dihydro-5-oxo-1H-imidazole-4-propanoic acid | ChEBI | | 4-Imidazolone-5-propanoate | ChEBI | | Imidazol-4-one-5-propionic acid | ChEBI | | 4,5-Dihydro-4-oxo-5-imidazolepropanoic acid | Generator | | 4,5-Dihydro-5-oxo-1H-imidazole-4-propanoate | Generator | | 4-Imidazolone-5-propanoic acid | Generator | | Imidazol-4-one-5-propionate | Generator | | 4-Imidazolone-5-propionate | Generator | | (S)-3-(5-oxo-4,5-Dihydro-3H-imidazol-4-yl)propanoate | HMDB | | (S)-3-(5-oxo-4,5-Dihydro-3H-imidazol-4-yl)propanoic acid | HMDB | | 3-(4-oxo-4,5-Dihydro-1H-imidazol-5-yl)propanoate | HMDB | | 3-(4-oxo-4,5-Dihydro-1H-imidazol-5-yl)propanoic acid | HMDB | | 3-(5-oxo-4,5-Dihydro-3H-imidazol-4-yl)propanoate | HMDB | | 3-(5-oxo-4,5-Dihydro-3H-imidazol-4-yl)propanoic acid | HMDB | | Imidazolone propionate | HMDB | | Imidazolone propionic acid | HMDB | | Imidazolonepropanoate | HMDB | | Imidazolonepropanoic acid | HMDB |
|
|---|
| Chemical Formula | C6H8N2O3 |
|---|
| Average Molecular Weight | 156.1393 |
|---|
| Monoisotopic Molecular Weight | 156.053492132 |
|---|
| IUPAC Name | 3-(5-oxo-4,5-dihydro-1H-imidazol-4-yl)propanoic acid |
|---|
| Traditional Name | 3-(5-oxo-1,4-dihydroimidazol-4-yl)propanoic acid |
|---|
| CAS Registry Number | 17340-16-8 |
|---|
| SMILES | OC(=O)CCC1N=CNC1=O |
|---|
| InChI Identifier | InChI=1S/C6H8N2O3/c9-5(10)2-1-4-6(11)8-3-7-4/h3-4H,1-2H2,(H,9,10)(H,7,8,11) |
|---|
| InChI Key | HEXMLHKQVUFYME-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as imidazolyl carboxylic acids and derivatives. These are organic compounds containing a carboxylic acid chain (of at least 2 carbon atoms) linked to an imidazole ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Azoles |
|---|
| Sub Class | Imidazoles |
|---|
| Direct Parent | Imidazolyl carboxylic acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Imidazolyl carboxylic acid derivative
- Carboxylic acid derivative
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Propargyl-type 1,3-dipolar organic compound
- Organic 1,3-dipolar compound
- Azacycle
- Carbonyl group
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Physiological effect | Not Available |
|---|
| Disposition | |
|---|
| Process | |
|---|
| Role | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Not Available |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Retention Times Underivatized| Chromatographic Method | Retention Time | Reference |
|---|
| Measured using a Waters Acquity ultraperformance liquid chromatography (UPLC) ethylene-bridged hybrid (BEH) C18 column (100 mm × 2.1 mm; 1.7 μmparticle diameter). Predicted by Afia on May 17, 2022. Predicted by Afia on May 17, 2022. | 0.83 minutes | 32390414 | | Predicted by Siyang on May 30, 2022 | 9.0164 minutes | 33406817 | | Predicted by Siyang using ReTip algorithm on June 8, 2022 | 6.78 minutes | 32390414 | | AjsUoB = Accucore 150 Amide HILIC with 10mM Ammonium Formate, 0.1% Formic Acid | 111.6 seconds | 40023050 | | Fem_Long = Waters ACQUITY UPLC HSS T3 C18 with Water:MeOH and 0.1% Formic Acid | 1071.4 seconds | 40023050 | | Fem_Lipids = Ascentis Express C18 with (60:40 water:ACN):(90:10 IPA:ACN) and 10mM NH4COOH + 0.1% Formic Acid | 298.0 seconds | 40023050 | | Life_Old = Waters ACQUITY UPLC BEH C18 with Water:(20:80 acetone:ACN) and 0.1% Formic Acid | 82.3 seconds | 40023050 | | Life_New = RP Waters ACQUITY UPLC HSS T3 C18 with Water:(30:70 MeOH:ACN) and 0.1% Formic Acid | 182.4 seconds | 40023050 | | RIKEN = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 66.9 seconds | 40023050 | | Eawag_XBridgeC18 = XBridge C18 3.5u 2.1x50 mm with Water:MeOH and 0.1% Formic Acid | 243.0 seconds | 40023050 | | BfG_NTS_RP1 =Agilent Zorbax Eclipse Plus C18 (2.1 mm x 150 mm, 3.5 um) with Water:ACN and 0.1% Formic Acid | 296.1 seconds | 40023050 | | HILIC_BDD_2 = Merck SeQuant ZIC-HILIC with ACN(0.1% formic acid):water(16 mM ammonium formate) | 130.5 seconds | 40023050 | | UniToyama_Atlantis = RP Waters Atlantis T3 (2.1 x 150 mm, 5 um) with ACN:Water and 0.1% Formic Acid | 635.7 seconds | 40023050 | | BDD_C18 = Hypersil Gold 1.9µm C18 with Water:ACN and 0.1% Formic Acid | 185.9 seconds | 40023050 | | UFZ_Phenomenex = Kinetex Core-Shell C18 2.6 um, 3.0 x 100 mm, Phenomenex with Water:MeOH and 0.1% Formic Acid | 882.9 seconds | 40023050 | | SNU_RIKEN_POS = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 199.7 seconds | 40023050 | | RPMMFDA = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 188.2 seconds | 40023050 | | MTBLS87 = Merck SeQuant ZIC-pHILIC column with ACN:Water and :ammonium carbonate | 597.8 seconds | 40023050 | | KI_GIAR_zic_HILIC_pH2_7 = Merck SeQuant ZIC-HILIC with ACN:Water and 0.1% FA | 206.3 seconds | 40023050 | | Meister zic-pHILIC pH9.3 = Merck SeQuant ZIC-pHILIC column with ACN:Water 5mM NH4Ac pH9.3 and 5mM ammonium acetate in water | 266.5 seconds | 40023050 |
Predicted Kovats Retention IndicesUnderivatizedDerivatized| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| 4-Imidazolone-5-propionic acid,1TMS,isomer #1 | C[Si](C)(C)OC(=O)CCC1N=CNC1=O | 1747.7 | Semi standard non polar | 33892256 | | 4-Imidazolone-5-propionic acid,1TMS,isomer #2 | C[Si](C)(C)N1C=NC(CCC(=O)O)C1=O | 1781.1 | Semi standard non polar | 33892256 | | 4-Imidazolone-5-propionic acid,2TMS,isomer #1 | C[Si](C)(C)OC(=O)CCC1N=CN([Si](C)(C)C)C1=O | 1826.2 | Semi standard non polar | 33892256 | | 4-Imidazolone-5-propionic acid,2TMS,isomer #1 | C[Si](C)(C)OC(=O)CCC1N=CN([Si](C)(C)C)C1=O | 1837.5 | Standard non polar | 33892256 | | 4-Imidazolone-5-propionic acid,2TMS,isomer #1 | C[Si](C)(C)OC(=O)CCC1N=CN([Si](C)(C)C)C1=O | 2768.3 | Standard polar | 33892256 | | 4-Imidazolone-5-propionic acid,1TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)CCC1N=CNC1=O | 1972.9 | Semi standard non polar | 33892256 | | 4-Imidazolone-5-propionic acid,1TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)N1C=NC(CCC(=O)O)C1=O | 2037.8 | Semi standard non polar | 33892256 | | 4-Imidazolone-5-propionic acid,2TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)CCC1N=CN([Si](C)(C)C(C)(C)C)C1=O | 2318.4 | Semi standard non polar | 33892256 | | 4-Imidazolone-5-propionic acid,2TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)CCC1N=CN([Si](C)(C)C(C)(C)C)C1=O | 2259.0 | Standard non polar | 33892256 | | 4-Imidazolone-5-propionic acid,2TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)CCC1N=CN([Si](C)(C)C(C)(C)C)C1=O | 2935.7 | Standard polar | 33892256 |
|
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - 4-Imidazolone-5-propionic acid GC-MS (Non-derivatized) - 70eV, Positive | splash10-03ec-9500000000-a11c5ca5f02078a53fc7 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 4-Imidazolone-5-propionic acid GC-MS (1 TMS) - 70eV, Positive | splash10-00di-8920000000-fed80cbe61ee26d1486a | 2017-10-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 4-Imidazolone-5-propionic acid GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 4-Imidazolone-5-propionic acid 10V, Positive-QTOF | splash10-000i-0900000000-36ccc12772d3e7b3bc01 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 4-Imidazolone-5-propionic acid 20V, Positive-QTOF | splash10-01p9-0900000000-8c7ad9614b1acbee7f96 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 4-Imidazolone-5-propionic acid 40V, Positive-QTOF | splash10-0f6x-9000000000-0561b28120ae734b99cf | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 4-Imidazolone-5-propionic acid 10V, Negative-QTOF | splash10-0a4i-0900000000-d24b3a3769f74c7e1858 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 4-Imidazolone-5-propionic acid 20V, Negative-QTOF | splash10-0a4u-8900000000-98a3f029124c94f1e6b1 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 4-Imidazolone-5-propionic acid 40V, Negative-QTOF | splash10-0006-9100000000-c75d14d5396894fff6f5 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 4-Imidazolone-5-propionic acid 10V, Negative-QTOF | splash10-0a4i-0900000000-c39bbd8fcbd13dc72dd1 | 2021-09-21 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 4-Imidazolone-5-propionic acid 20V, Negative-QTOF | splash10-08gl-8900000000-9ecbb63abc1319562a12 | 2021-09-21 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 4-Imidazolone-5-propionic acid 40V, Negative-QTOF | splash10-000x-9000000000-50c3ee9255335f634b30 | 2021-09-21 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 4-Imidazolone-5-propionic acid 10V, Positive-QTOF | splash10-0bt9-0900000000-75e80efea901b323015e | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 4-Imidazolone-5-propionic acid 20V, Positive-QTOF | splash10-03di-6900000000-a2d551ead7379a1c56b7 | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 4-Imidazolone-5-propionic acid 40V, Positive-QTOF | splash10-0006-9100000000-d2613558e32279e944b3 | 2021-09-25 | Wishart Lab | View Spectrum |
IR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M-H]-) | 2023-02-03 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M+H]+) | 2023-02-03 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M+Na]+) | 2023-02-03 | FELIX lab | View Spectrum |
|
|---|