| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Detected and Quantified |
|---|
| Creation Date | 2005-11-16 15:48:42 UTC |
|---|
| Update Date | 2023-02-21 17:15:27 UTC |
|---|
| HMDB ID | HMDB0001123 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 2-Aminobenzoic acid |
|---|
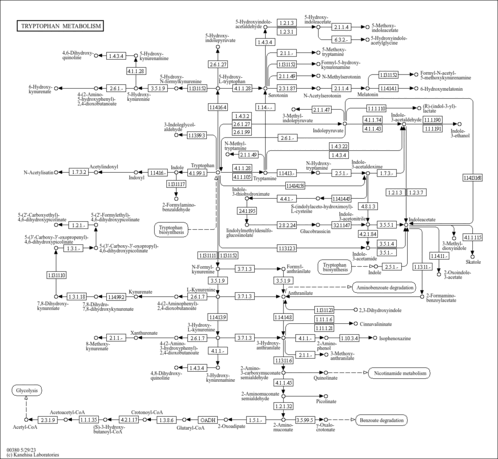
| Description | 2-Aminobenzoic acid, also known as anthranilic acid or O-aminobenzoate, belongs to the class of organic compounds known as aminobenzoic acids. These are benzoic acids containing an amine group attached to the benzene moiety. Within humans, 2-aminobenzoic acid participates in a number of enzymatic reactions. In particular, 2-aminobenzoic acid and formic acid can be biosynthesized from formylanthranilic acid through its interaction with the enzyme kynurenine formamidase. In addition, 2-aminobenzoic acid and L-alanine can be biosynthesized from L-kynurenine through its interaction with the enzyme kynureninase. It is a substrate of enzyme 2-Aminobenzoic acid hydroxylase in benzoate degradation via hydroxylation pathway (KEGG). In humans, 2-aminobenzoic acid is involved in tryptophan metabolism. Outside of the human body, 2-Aminobenzoic acid has been detected, but not quantified in several different foods, such as mamey sapotes, prairie turnips, rowals, natal plums, and hyacinth beans. This could make 2-aminobenzoic acid a potential biomarker for the consumption of these foods. 2-Aminobenzoic acid is a is a tryptophan-derived uremic toxin with multidirectional properties that can affect the hemostatic system. Uremic syndrome may affect any part of the body and can cause nausea, vomiting, loss of appetite, and weight loss. Chronic exposure of uremic toxins can lead to a number of conditions including renal damage, chronic kidney disease and cardiovascular disease. It can also cause changes in mental status, such as confusion, reduced awareness, agitation, psychosis, seizures, and coma. |
|---|
| Structure | InChI=1S/C7H7NO2/c8-6-4-2-1-3-5(6)7(9)10/h1-4H,8H2,(H,9,10) |
|---|
| Synonyms | | Value | Source |
|---|
| 2-Aminobenzoesaeure | ChEBI | | 2-Carboxyaniline | ChEBI | | O-Aminobenzoesaeure | ChEBI | | O-Aminobenzoic acid | ChEBI | | O-Carboxyaniline | ChEBI | | Vitamin L1 | ChEBI | | Anthranilic acid | Kegg | | 2-Aminobenzoate | Kegg | | O-Aminobenzoate | Generator | | Anthranilate | Generator | | Anthranilic acid, cadmium salt | HMDB | | Anthranilic acid, calcium (2:1) salt | HMDB | | Anthranilic acid, monolithium salt | HMDB | | Sodium anthranilate | HMDB | | Anthranilic acid, dihydrochloride | HMDB | | Anthranilic acid, hydrochloride | HMDB | | Anthranilic acid, monosodium salt | HMDB | | 1-Amino-2-carboxybenzene | HMDB | | 2-Amino-benzoate | HMDB | | 2-Amino-benzoic acid | HMDB | | 2-Aminophenylacetate | HMDB | | 2-Aminophenylacetic acid | HMDB | | Anthranate | HMDB | | Anthranic acid | HMDB | | Anthranilic acid GR | HMDB | | Carboxyaniline | HMDB | | H-2-Abz-OH | HMDB | | Kyselina anthranilova | HMDB | | Kyselina O-aminobenzoova | HMDB | | O-Amino-benzoate | HMDB | | O-Amino-benzoic acid | HMDB | | O-Anthranilate | HMDB | | O-Anthranilic acid | HMDB | | Ortho-amidobenzoate | HMDB | | Ortho-amidobenzoic acid | HMDB | | Ortho-aminobenzoate | HMDB | | Ortho-aminobenzoic acid | HMDB | | Vitamin L | HMDB |
|
|---|
| Chemical Formula | C7H7NO2 |
|---|
| Average Molecular Weight | 137.136 |
|---|
| Monoisotopic Molecular Weight | 137.047678473 |
|---|
| IUPAC Name | 2-aminobenzoic acid |
|---|
| Traditional Name | 2-aminobenzoic acid |
|---|
| CAS Registry Number | 118-92-3 |
|---|
| SMILES | NC1=CC=CC=C1C(O)=O |
|---|
| InChI Identifier | InChI=1S/C7H7NO2/c8-6-4-2-1-3-5(6)7(9)10/h1-4H,8H2,(H,9,10) |
|---|
| InChI Key | RWZYAGGXGHYGMB-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as aminobenzoic acids. These are benzoic acids containing an amine group attached to the benzene moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Benzoic acids and derivatives |
|---|
| Direct Parent | Aminobenzoic acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Aminobenzoic acid
- Benzoic acid
- Benzoyl
- Aniline or substituted anilines
- Vinylogous amide
- Amino acid or derivatives
- Amino acid
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxide
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Amine
- Hydrocarbon derivative
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Physiological effect | |
|---|
| Disposition | |
|---|
| Process | |
|---|
| Role | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | 146.5 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 3.5 mg/mL | Not Available | | LogP | 1.21 | HANSCH,C ET AL. (1995) |
|
|---|
| Experimental Chromatographic Properties | Experimental Collision Cross Sections |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Retention Times Underivatized| Chromatographic Method | Retention Time | Reference |
|---|
| Measured using a Waters Acquity ultraperformance liquid chromatography (UPLC) ethylene-bridged hybrid (BEH) C18 column (100 mm × 2.1 mm; 1.7 μmparticle diameter). Predicted by Afia on May 17, 2022. Predicted by Afia on May 17, 2022. | 2.38 minutes | 32390414 | | Predicted by Siyang on May 30, 2022 | 10.3603 minutes | 33406817 | | Predicted by Siyang using ReTip algorithm on June 8, 2022 | 4.0 minutes | 32390414 | | AjsUoB = Accucore 150 Amide HILIC with 10mM Ammonium Formate, 0.1% Formic Acid | 60.6 seconds | 40023050 | | Fem_Long = Waters ACQUITY UPLC HSS T3 C18 with Water:MeOH and 0.1% Formic Acid | 1062.5 seconds | 40023050 | | Fem_Lipids = Ascentis Express C18 with (60:40 water:ACN):(90:10 IPA:ACN) and 10mM NH4COOH + 0.1% Formic Acid | 351.0 seconds | 40023050 | | Life_Old = Waters ACQUITY UPLC BEH C18 with Water:(20:80 acetone:ACN) and 0.1% Formic Acid | 81.9 seconds | 40023050 | | Life_New = RP Waters ACQUITY UPLC HSS T3 C18 with Water:(30:70 MeOH:ACN) and 0.1% Formic Acid | 211.6 seconds | 40023050 | | RIKEN = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 70.9 seconds | 40023050 | | Eawag_XBridgeC18 = XBridge C18 3.5u 2.1x50 mm with Water:MeOH and 0.1% Formic Acid | 280.3 seconds | 40023050 | | BfG_NTS_RP1 =Agilent Zorbax Eclipse Plus C18 (2.1 mm x 150 mm, 3.5 um) with Water:ACN and 0.1% Formic Acid | 347.3 seconds | 40023050 | | HILIC_BDD_2 = Merck SeQuant ZIC-HILIC with ACN(0.1% formic acid):water(16 mM ammonium formate) | 148.5 seconds | 40023050 | | UniToyama_Atlantis = RP Waters Atlantis T3 (2.1 x 150 mm, 5 um) with ACN:Water and 0.1% Formic Acid | 755.5 seconds | 40023050 | | BDD_C18 = Hypersil Gold 1.9µm C18 with Water:ACN and 0.1% Formic Acid | 201.3 seconds | 40023050 | | UFZ_Phenomenex = Kinetex Core-Shell C18 2.6 um, 3.0 x 100 mm, Phenomenex with Water:MeOH and 0.1% Formic Acid | 930.1 seconds | 40023050 | | SNU_RIKEN_POS = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 229.5 seconds | 40023050 | | RPMMFDA = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 301.9 seconds | 40023050 | | MTBLS87 = Merck SeQuant ZIC-pHILIC column with ACN:Water and :ammonium carbonate | 473.8 seconds | 40023050 | | KI_GIAR_zic_HILIC_pH2_7 = Merck SeQuant ZIC-HILIC with ACN:Water and 0.1% FA | 213.7 seconds | 40023050 | | Meister zic-pHILIC pH9.3 = Merck SeQuant ZIC-pHILIC column with ACN:Water 5mM NH4Ac pH9.3 and 5mM ammonium acetate in water | 178.3 seconds | 40023050 |
Predicted Kovats Retention IndicesUnderivatizedDerivatized| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| 2-Aminobenzoic acid,1TMS,isomer #1 | C[Si](C)(C)OC(=O)C1=CC=CC=C1N | 1498.0 | Semi standard non polar | 33892256 | | 2-Aminobenzoic acid,1TMS,isomer #2 | C[Si](C)(C)NC1=CC=CC=C1C(=O)O | 1607.4 | Semi standard non polar | 33892256 | | 2-Aminobenzoic acid,2TMS,isomer #1 | C[Si](C)(C)NC1=CC=CC=C1C(=O)O[Si](C)(C)C | 1600.8 | Semi standard non polar | 33892256 | | 2-Aminobenzoic acid,2TMS,isomer #1 | C[Si](C)(C)NC1=CC=CC=C1C(=O)O[Si](C)(C)C | 1634.8 | Standard non polar | 33892256 | | 2-Aminobenzoic acid,2TMS,isomer #1 | C[Si](C)(C)NC1=CC=CC=C1C(=O)O[Si](C)(C)C | 1778.7 | Standard polar | 33892256 | | 2-Aminobenzoic acid,2TMS,isomer #2 | C[Si](C)(C)N(C1=CC=CC=C1C(=O)O)[Si](C)(C)C | 1660.6 | Semi standard non polar | 33892256 | | 2-Aminobenzoic acid,2TMS,isomer #2 | C[Si](C)(C)N(C1=CC=CC=C1C(=O)O)[Si](C)(C)C | 1772.2 | Standard non polar | 33892256 | | 2-Aminobenzoic acid,2TMS,isomer #2 | C[Si](C)(C)N(C1=CC=CC=C1C(=O)O)[Si](C)(C)C | 1839.3 | Standard polar | 33892256 | | 2-Aminobenzoic acid,3TMS,isomer #1 | C[Si](C)(C)OC(=O)C1=CC=CC=C1N([Si](C)(C)C)[Si](C)(C)C | 1633.8 | Semi standard non polar | 33892256 | | 2-Aminobenzoic acid,3TMS,isomer #1 | C[Si](C)(C)OC(=O)C1=CC=CC=C1N([Si](C)(C)C)[Si](C)(C)C | 1731.4 | Standard non polar | 33892256 | | 2-Aminobenzoic acid,3TMS,isomer #1 | C[Si](C)(C)OC(=O)C1=CC=CC=C1N([Si](C)(C)C)[Si](C)(C)C | 1712.5 | Standard polar | 33892256 | | 2-Aminobenzoic acid,1TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)C1=CC=CC=C1N | 1747.5 | Semi standard non polar | 33892256 | | 2-Aminobenzoic acid,1TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)NC1=CC=CC=C1C(=O)O | 1858.5 | Semi standard non polar | 33892256 | | 2-Aminobenzoic acid,2TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)NC1=CC=CC=C1C(=O)O[Si](C)(C)C(C)(C)C | 2045.2 | Semi standard non polar | 33892256 | | 2-Aminobenzoic acid,2TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)NC1=CC=CC=C1C(=O)O[Si](C)(C)C(C)(C)C | 2031.4 | Standard non polar | 33892256 | | 2-Aminobenzoic acid,2TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)NC1=CC=CC=C1C(=O)O[Si](C)(C)C(C)(C)C | 2087.6 | Standard polar | 33892256 | | 2-Aminobenzoic acid,2TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)N(C1=CC=CC=C1C(=O)O)[Si](C)(C)C(C)(C)C | 2085.5 | Semi standard non polar | 33892256 | | 2-Aminobenzoic acid,2TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)N(C1=CC=CC=C1C(=O)O)[Si](C)(C)C(C)(C)C | 2140.7 | Standard non polar | 33892256 | | 2-Aminobenzoic acid,2TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)N(C1=CC=CC=C1C(=O)O)[Si](C)(C)C(C)(C)C | 2058.6 | Standard polar | 33892256 | | 2-Aminobenzoic acid,3TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)C1=CC=CC=C1N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2278.8 | Semi standard non polar | 33892256 | | 2-Aminobenzoic acid,3TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)C1=CC=CC=C1N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2301.9 | Standard non polar | 33892256 | | 2-Aminobenzoic acid,3TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)C1=CC=CC=C1N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2116.6 | Standard polar | 33892256 |
|
|---|
| Disease References | | Uremia |
|---|
- Duranton F, Cohen G, De Smet R, Rodriguez M, Jankowski J, Vanholder R, Argiles A: Normal and pathologic concentrations of uremic toxins. J Am Soc Nephrol. 2012 Jul;23(7):1258-70. doi: 10.1681/ASN.2011121175. Epub 2012 May 24. [PubMed:22626821 ]
| | Colorectal cancer |
|---|
- Sinha R, Ahn J, Sampson JN, Shi J, Yu G, Xiong X, Hayes RB, Goedert JJ: Fecal Microbiota, Fecal Metabolome, and Colorectal Cancer Interrelations. PLoS One. 2016 Mar 25;11(3):e0152126. doi: 10.1371/journal.pone.0152126. eCollection 2016. [PubMed:27015276 ]
- Goedert JJ, Sampson JN, Moore SC, Xiao Q, Xiong X, Hayes RB, Ahn J, Shi J, Sinha R: Fecal metabolomics: assay performance and association with colorectal cancer. Carcinogenesis. 2014 Sep;35(9):2089-96. doi: 10.1093/carcin/bgu131. Epub 2014 Jul 18. [PubMed:25037050 ]
| | Eosinophilic esophagitis |
|---|
- Slae, M., Huynh, H., Wishart, D.S. (2014). Analysis of 30 normal pediatric urine samples via NMR spectroscopy (unpublished work). NA.
|
|
|---|
| General References | - Igari T, Tsuchizawa M, Shimamura T: Alteration of tryptophan metabolism in the synovial fluid of patients with rheumatoid arthritis and osteoarthritis. Tohoku J Exp Med. 1987 Oct;153(2):79-86. [PubMed:3500530 ]
- Calandra P: Research on tryptophan metabolites "via kynurenine" in epidermis of man and mouse. Acta Vitaminol Enzymol. 1975;29(1-6):158-60. [PubMed:1244085 ]
- Di Marco GS, Quinto BM, Juliano M, Carmona AK, Stella RC, Plavnik FL, Casarini DE: Purification and characterization of a neutral endopeptidase-like enzyme from human urine. J Hypertens. 1998 Dec;16(12 Pt 2):1971-8. [PubMed:9886885 ]
- Hagag N, Birnbaum ER, Darnall DW: Resonance energy transfer between cysteine-34, tryptophan-214, and tyrosine-411 of human serum albumin. Biochemistry. 1983 May 10;22(10):2420-7. [PubMed:6860638 ]
- Little CH, Georgiou GM, Shelton MJ, Simpson F, Cone RE: Clinical and immunological responses in subjects sensitive to solvents. Arch Environ Health. 1999 Jan-Feb;54(1):6-14. [PubMed:10025410 ]
- Ritchie MR, Morton MS, Thompson AM, Deighton N, Blake A, Cummings JH, Steel CM: Investigation of the reliability of 24 h urine excretion as a biomarker of isoflavone exposure over time and over a wide range of isoflavone intakes. Eur J Clin Nutr. 2004 Sep;58(9):1286-9. [PubMed:15054404 ]
- Ortega RM, Andres P, Martinez RM, Lopez-Sobaler AM: Vitamin A status during the third trimester of pregnancy in Spanish women: influence on concentrations of vitamin A in breast milk. Am J Clin Nutr. 1997 Sep;66(3):564-8. [PubMed:9280174 ]
- Alves MF, Araujo MC, Juliano MA, Oliveira EM, Krieger JE, Casarini DE, Juliano L, Carmona AK: A continuous fluorescent assay for the determination of plasma and tissue angiotensin I-converting enzyme activity. Braz J Med Biol Res. 2005 Jun;38(6):861-8. Epub 2005 Jun 1. [PubMed:15933779 ]
- Soma J, Sugawara T, Huang YD, Nakajima J, Kawamura M: Tranilast slows the progression of advanced diabetic nephropathy. Nephron. 2002;92(3):693-8. [PubMed:12372957 ]
- Ahmad S: The functional roles of cytochrome P-450 mediated systems: present knowledge and future areas of investigations. Drug Metab Rev. 1979;10(1):1-14. [PubMed:118858 ]
- Spivak W, Carey MC: Reverse-phase h.p.l.c. separation, quantification and preparation of bilirubin and its conjugates from native bile. Quantitative analysis of the intact tetrapyrroles based on h.p.l.c. of their ethyl anthranilate azo derivatives. Biochem J. 1985 Feb 1;225(3):787-805. [PubMed:3919713 ]
- Duranton F, Cohen G, De Smet R, Rodriguez M, Jankowski J, Vanholder R, Argiles A: Normal and pathologic concentrations of uremic toxins. J Am Soc Nephrol. 2012 Jul;23(7):1258-70. doi: 10.1681/ASN.2011121175. Epub 2012 May 24. [PubMed:22626821 ]
|
|---|