| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Detected but not Quantified |
|---|
| Creation Date | 2005-11-16 15:48:42 UTC |
|---|
| Update Date | 2022-03-07 02:49:08 UTC |
|---|
| HMDB ID | HMDB0001129 |
|---|
| Secondary Accession Numbers | - HMDB0062724
- HMDB01129
- HMDB62724
|
|---|
| Metabolite Identification |
|---|
| Common Name | N-Acetylmannosamine |
|---|
| Description | N-Acetylmannosamine, also known as beta-ManNAcc or β-ManNAc, belongs to the class of organic compounds known as acylaminosugars. These are organic compounds containing a sugar linked to a chain through N-acyl group. Within humans, N-acetylmannosamine participates in a number of enzymatic reactions. In particular, N-acetylmannosamine can be biosynthesized from N-acetyl-D-glucosamine, which is catalyzed by the enzyme N-acylglucosamine 2-epimerase. In addition, N-acetylmannosamine and uridine 5'-diphosphate can be biosynthesized from uridine diphosphate-N-acetylglucosamine; which is mediated by the enzyme bifunctional UDP-N-acetyl glucosamine 2-epimerase / N-acetylmannosamine kinase. In humans, N-acetylmannosamine is involved in the metabolic disorder called the salla disease/infantile sialic acid storage disease pathway. In the rate-limiting step of the pathway, UDP-GlcNAc is converted into ManNAc by UDP-GlcNAc 2-epimerase, encoded by the epimerase domain of GNE. Improved sialylation after the addition of ManNAc and other supporting ingredients to the culture medium not only increases manufacturing yield, but also improves therapeutic efficacy by increasing solubility, increasing half-life and reducing immunogenicity by reducing the formation of antibodies to the therapeutic glycoprotein When the GNE epimerase kinase does not function correctly in the human body thereby reducing the available ManNAc, it is reasonable to assume that treatment with ManNAc could assist with improving health benefits. There is no available therapy to treat GNE myopathy. ManNAc is the first committed biological precursor of N-acetylneuraminic acid (Neu5Ac, sialic acid). |
|---|
| Structure | CC(=O)N[C@@H]1[C@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O InChI=1S/C8H15NO6/c1-3(11)9-5-7(13)6(12)4(2-10)15-8(5)14/h4-8,10,12-14H,2H2,1H3,(H,9,11)/t4-,5+,6-,7-,8-/m1/s1 |
|---|
| Synonyms | | Value | Source |
|---|
| beta-ManNAc | ChEBI | | N-Acetyl-beta-mannosamine | ChEBI | | b-ManNAc | Generator | | Β-mannac | Generator | | N-Acetyl-b-mannosamine | Generator | | N-Acetyl-β-mannosamine | Generator | | 2-Acetamido-2-deoxy-D-mannopyranose | HMDB | | 2-Acetamido-2-deoxy-D-mannose | HMDB | | ManNAc | HMDB | | N-Acetyl-D-mannosamine | HMDB | | N-Acetylmannosamine, (D)-isomer | HMDB | | N-Acetylmannosamine, (L)-isomer | HMDB |
|
|---|
| Chemical Formula | C8H15NO6 |
|---|
| Average Molecular Weight | 221.2078 |
|---|
| Monoisotopic Molecular Weight | 221.089937217 |
|---|
| IUPAC Name | N-[(2R,3S,4R,5S,6R)-2,4,5-trihydroxy-6-(hydroxymethyl)oxan-3-yl]acetamide |
|---|
| Traditional Name | N-acetylmannosamine |
|---|
| CAS Registry Number | 7772-94-3 |
|---|
| SMILES | CC(=O)N[C@@H]1[C@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O |
|---|
| InChI Identifier | InChI=1S/C8H15NO6/c1-3(11)9-5-7(13)6(12)4(2-10)15-8(5)14/h4-8,10,12-14H,2H2,1H3,(H,9,11)/t4-,5+,6-,7-,8-/m1/s1 |
|---|
| InChI Key | OVRNDRQMDRJTHS-OZRXBMAMSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as acylaminosugars. These are organic compounds containing a sugar linked to a chain through N-acyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Acylaminosugars |
|---|
| Alternative Parents | |
|---|
| Substituents | - Acylaminosugar
- N-acyl-alpha-hexosamine
- Hexose monosaccharide
- Monosaccharide
- Oxane
- Acetamide
- Carboxamide group
- Hemiacetal
- Secondary carboxylic acid amide
- Secondary alcohol
- Carboxylic acid derivative
- Oxacycle
- Organoheterocyclic compound
- Polyol
- Alcohol
- Hydrocarbon derivative
- Organic oxide
- Organic nitrogen compound
- Organopnictogen compound
- Organonitrogen compound
- Primary alcohol
- Carbonyl group
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Physiological effect | Not Available |
|---|
| Disposition | |
|---|
| Process | |
|---|
| Role | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | -3.22 | Extrapolated |
|
|---|
| Experimental Chromatographic Properties | Not Available |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Kovats Retention IndicesUnderivatizedDerivatized| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| N-Acetylmannosamine,1TMS,isomer #1 | CC(=O)N[C@@H]1[C@H](O[Si](C)(C)C)O[C@H](CO)[C@@H](O)[C@@H]1O | 1894.9 | Semi standard non polar | 33892256 | | N-Acetylmannosamine,1TMS,isomer #2 | CC(=O)N[C@@H]1[C@H](O)O[C@H](CO[Si](C)(C)C)[C@@H](O)[C@@H]1O | 1900.1 | Semi standard non polar | 33892256 | | N-Acetylmannosamine,1TMS,isomer #3 | CC(=O)N[C@@H]1[C@H](O)O[C@H](CO)[C@@H](O[Si](C)(C)C)[C@@H]1O | 1898.0 | Semi standard non polar | 33892256 | | N-Acetylmannosamine,1TMS,isomer #4 | CC(=O)N[C@@H]1[C@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O[Si](C)(C)C | 1908.2 | Semi standard non polar | 33892256 | | N-Acetylmannosamine,1TMS,isomer #5 | CC(=O)N([C@@H]1[C@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O)[Si](C)(C)C | 1835.0 | Semi standard non polar | 33892256 | | N-Acetylmannosamine,2TMS,isomer #1 | CC(=O)N[C@@H]1[C@H](O[Si](C)(C)C)O[C@H](CO[Si](C)(C)C)[C@@H](O)[C@@H]1O | 1915.8 | Semi standard non polar | 33892256 | | N-Acetylmannosamine,2TMS,isomer #10 | CC(=O)N([C@@H]1[C@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O[Si](C)(C)C)[Si](C)(C)C | 1897.1 | Semi standard non polar | 33892256 | | N-Acetylmannosamine,2TMS,isomer #2 | CC(=O)N[C@@H]1[C@H](O[Si](C)(C)C)O[C@H](CO)[C@@H](O[Si](C)(C)C)[C@@H]1O | 1930.3 | Semi standard non polar | 33892256 | | N-Acetylmannosamine,2TMS,isomer #3 | CC(=O)N[C@@H]1[C@H](O[Si](C)(C)C)O[C@H](CO)[C@@H](O)[C@@H]1O[Si](C)(C)C | 1938.6 | Semi standard non polar | 33892256 | | N-Acetylmannosamine,2TMS,isomer #4 | CC(=O)N([C@@H]1[C@H](O[Si](C)(C)C)O[C@H](CO)[C@@H](O)[C@@H]1O)[Si](C)(C)C | 1890.6 | Semi standard non polar | 33892256 | | N-Acetylmannosamine,2TMS,isomer #5 | CC(=O)N[C@@H]1[C@H](O)O[C@H](CO[Si](C)(C)C)[C@@H](O[Si](C)(C)C)[C@@H]1O | 1939.6 | Semi standard non polar | 33892256 | | N-Acetylmannosamine,2TMS,isomer #6 | CC(=O)N[C@@H]1[C@H](O)O[C@H](CO[Si](C)(C)C)[C@@H](O)[C@@H]1O[Si](C)(C)C | 1939.6 | Semi standard non polar | 33892256 | | N-Acetylmannosamine,2TMS,isomer #7 | CC(=O)N([C@@H]1[C@H](O)O[C@H](CO[Si](C)(C)C)[C@@H](O)[C@@H]1O)[Si](C)(C)C | 1911.9 | Semi standard non polar | 33892256 | | N-Acetylmannosamine,2TMS,isomer #8 | CC(=O)N[C@@H]1[C@H](O)O[C@H](CO)[C@@H](O[Si](C)(C)C)[C@@H]1O[Si](C)(C)C | 1933.2 | Semi standard non polar | 33892256 | | N-Acetylmannosamine,2TMS,isomer #9 | CC(=O)N([C@@H]1[C@H](O)O[C@H](CO)[C@@H](O[Si](C)(C)C)[C@@H]1O)[Si](C)(C)C | 1895.9 | Semi standard non polar | 33892256 | | N-Acetylmannosamine,3TMS,isomer #1 | CC(=O)N[C@@H]1[C@H](O[Si](C)(C)C)O[C@H](CO[Si](C)(C)C)[C@@H](O[Si](C)(C)C)[C@@H]1O | 2003.5 | Semi standard non polar | 33892256 | | N-Acetylmannosamine,3TMS,isomer #10 | CC(=O)N([C@@H]1[C@H](O)O[C@H](CO)[C@@H](O[Si](C)(C)C)[C@@H]1O[Si](C)(C)C)[Si](C)(C)C | 1967.8 | Semi standard non polar | 33892256 | | N-Acetylmannosamine,3TMS,isomer #2 | CC(=O)N[C@@H]1[C@H](O[Si](C)(C)C)O[C@H](CO[Si](C)(C)C)[C@@H](O)[C@@H]1O[Si](C)(C)C | 2011.5 | Semi standard non polar | 33892256 | | N-Acetylmannosamine,3TMS,isomer #3 | CC(=O)N([C@@H]1[C@H](O[Si](C)(C)C)O[C@H](CO[Si](C)(C)C)[C@@H](O)[C@@H]1O)[Si](C)(C)C | 1939.5 | Semi standard non polar | 33892256 | | N-Acetylmannosamine,3TMS,isomer #4 | CC(=O)N[C@@H]1[C@H](O[Si](C)(C)C)O[C@H](CO)[C@@H](O[Si](C)(C)C)[C@@H]1O[Si](C)(C)C | 2016.7 | Semi standard non polar | 33892256 | | N-Acetylmannosamine,3TMS,isomer #5 | CC(=O)N([C@@H]1[C@H](O[Si](C)(C)C)O[C@H](CO)[C@@H](O[Si](C)(C)C)[C@@H]1O)[Si](C)(C)C | 1961.8 | Semi standard non polar | 33892256 | | N-Acetylmannosamine,3TMS,isomer #6 | CC(=O)N([C@@H]1[C@H](O[Si](C)(C)C)O[C@H](CO)[C@@H](O)[C@@H]1O[Si](C)(C)C)[Si](C)(C)C | 1981.0 | Semi standard non polar | 33892256 | | N-Acetylmannosamine,3TMS,isomer #7 | CC(=O)N[C@@H]1[C@H](O)O[C@H](CO[Si](C)(C)C)[C@@H](O[Si](C)(C)C)[C@@H]1O[Si](C)(C)C | 2006.4 | Semi standard non polar | 33892256 | | N-Acetylmannosamine,3TMS,isomer #8 | CC(=O)N([C@@H]1[C@H](O)O[C@H](CO[Si](C)(C)C)[C@@H](O[Si](C)(C)C)[C@@H]1O)[Si](C)(C)C | 1967.9 | Semi standard non polar | 33892256 | | N-Acetylmannosamine,3TMS,isomer #9 | CC(=O)N([C@@H]1[C@H](O)O[C@H](CO[Si](C)(C)C)[C@@H](O)[C@@H]1O[Si](C)(C)C)[Si](C)(C)C | 1970.5 | Semi standard non polar | 33892256 | | N-Acetylmannosamine,4TMS,isomer #1 | CC(=O)N[C@@H]1[C@H](O[Si](C)(C)C)O[C@H](CO[Si](C)(C)C)[C@@H](O[Si](C)(C)C)[C@@H]1O[Si](C)(C)C | 2036.2 | Semi standard non polar | 33892256 | | N-Acetylmannosamine,4TMS,isomer #2 | CC(=O)N([C@@H]1[C@H](O[Si](C)(C)C)O[C@H](CO[Si](C)(C)C)[C@@H](O[Si](C)(C)C)[C@@H]1O)[Si](C)(C)C | 2024.8 | Semi standard non polar | 33892256 | | N-Acetylmannosamine,4TMS,isomer #3 | CC(=O)N([C@@H]1[C@H](O[Si](C)(C)C)O[C@H](CO[Si](C)(C)C)[C@@H](O)[C@@H]1O[Si](C)(C)C)[Si](C)(C)C | 2040.9 | Semi standard non polar | 33892256 | | N-Acetylmannosamine,4TMS,isomer #4 | CC(=O)N([C@@H]1[C@H](O[Si](C)(C)C)O[C@H](CO)[C@@H](O[Si](C)(C)C)[C@@H]1O[Si](C)(C)C)[Si](C)(C)C | 2039.6 | Semi standard non polar | 33892256 | | N-Acetylmannosamine,4TMS,isomer #5 | CC(=O)N([C@@H]1[C@H](O)O[C@H](CO[Si](C)(C)C)[C@@H](O[Si](C)(C)C)[C@@H]1O[Si](C)(C)C)[Si](C)(C)C | 2031.3 | Semi standard non polar | 33892256 | | N-Acetylmannosamine,5TMS,isomer #1 | CC(=O)N([C@@H]1[C@H](O[Si](C)(C)C)O[C@H](CO[Si](C)(C)C)[C@@H](O[Si](C)(C)C)[C@@H]1O[Si](C)(C)C)[Si](C)(C)C | 2092.3 | Semi standard non polar | 33892256 | | N-Acetylmannosamine,5TMS,isomer #1 | CC(=O)N([C@@H]1[C@H](O[Si](C)(C)C)O[C@H](CO[Si](C)(C)C)[C@@H](O[Si](C)(C)C)[C@@H]1O[Si](C)(C)C)[Si](C)(C)C | 2154.1 | Standard non polar | 33892256 | | N-Acetylmannosamine,5TMS,isomer #1 | CC(=O)N([C@@H]1[C@H](O[Si](C)(C)C)O[C@H](CO[Si](C)(C)C)[C@@H](O[Si](C)(C)C)[C@@H]1O[Si](C)(C)C)[Si](C)(C)C | 2100.6 | Standard polar | 33892256 | | N-Acetylmannosamine,1TBDMS,isomer #1 | CC(=O)N[C@@H]1[C@H](O[Si](C)(C)C(C)(C)C)O[C@H](CO)[C@@H](O)[C@@H]1O | 2151.2 | Semi standard non polar | 33892256 | | N-Acetylmannosamine,1TBDMS,isomer #2 | CC(=O)N[C@@H]1[C@H](O)O[C@H](CO[Si](C)(C)C(C)(C)C)[C@@H](O)[C@@H]1O | 2148.3 | Semi standard non polar | 33892256 | | N-Acetylmannosamine,1TBDMS,isomer #3 | CC(=O)N[C@@H]1[C@H](O)O[C@H](CO)[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O | 2164.7 | Semi standard non polar | 33892256 | | N-Acetylmannosamine,1TBDMS,isomer #4 | CC(=O)N[C@@H]1[C@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O[Si](C)(C)C(C)(C)C | 2166.9 | Semi standard non polar | 33892256 | | N-Acetylmannosamine,1TBDMS,isomer #5 | CC(=O)N([C@@H]1[C@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O)[Si](C)(C)C(C)(C)C | 2109.9 | Semi standard non polar | 33892256 | | N-Acetylmannosamine,2TBDMS,isomer #1 | CC(=O)N[C@@H]1[C@H](O[Si](C)(C)C(C)(C)C)O[C@H](CO[Si](C)(C)C(C)(C)C)[C@@H](O)[C@@H]1O | 2423.5 | Semi standard non polar | 33892256 | | N-Acetylmannosamine,2TBDMS,isomer #10 | CC(=O)N([C@@H]1[C@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2384.2 | Semi standard non polar | 33892256 | | N-Acetylmannosamine,2TBDMS,isomer #2 | CC(=O)N[C@@H]1[C@H](O[Si](C)(C)C(C)(C)C)O[C@H](CO)[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O | 2439.8 | Semi standard non polar | 33892256 | | N-Acetylmannosamine,2TBDMS,isomer #3 | CC(=O)N[C@@H]1[C@H](O[Si](C)(C)C(C)(C)C)O[C@H](CO)[C@@H](O)[C@@H]1O[Si](C)(C)C(C)(C)C | 2433.2 | Semi standard non polar | 33892256 | | N-Acetylmannosamine,2TBDMS,isomer #4 | CC(=O)N([C@@H]1[C@H](O[Si](C)(C)C(C)(C)C)O[C@H](CO)[C@@H](O)[C@@H]1O)[Si](C)(C)C(C)(C)C | 2371.0 | Semi standard non polar | 33892256 | | N-Acetylmannosamine,2TBDMS,isomer #5 | CC(=O)N[C@@H]1[C@H](O)O[C@H](CO[Si](C)(C)C(C)(C)C)[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O | 2427.0 | Semi standard non polar | 33892256 | | N-Acetylmannosamine,2TBDMS,isomer #6 | CC(=O)N[C@@H]1[C@H](O)O[C@H](CO[Si](C)(C)C(C)(C)C)[C@@H](O)[C@@H]1O[Si](C)(C)C(C)(C)C | 2436.0 | Semi standard non polar | 33892256 | | N-Acetylmannosamine,2TBDMS,isomer #7 | CC(=O)N([C@@H]1[C@H](O)O[C@H](CO[Si](C)(C)C(C)(C)C)[C@@H](O)[C@@H]1O)[Si](C)(C)C(C)(C)C | 2406.0 | Semi standard non polar | 33892256 | | N-Acetylmannosamine,2TBDMS,isomer #8 | CC(=O)N[C@@H]1[C@H](O)O[C@H](CO)[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O[Si](C)(C)C(C)(C)C | 2423.5 | Semi standard non polar | 33892256 | | N-Acetylmannosamine,2TBDMS,isomer #9 | CC(=O)N([C@@H]1[C@H](O)O[C@H](CO)[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O)[Si](C)(C)C(C)(C)C | 2389.3 | Semi standard non polar | 33892256 | | N-Acetylmannosamine,3TBDMS,isomer #1 | CC(=O)N[C@@H]1[C@H](O[Si](C)(C)C(C)(C)C)O[C@H](CO[Si](C)(C)C(C)(C)C)[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O | 2686.1 | Semi standard non polar | 33892256 | | N-Acetylmannosamine,3TBDMS,isomer #10 | CC(=O)N([C@@H]1[C@H](O)O[C@H](CO)[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2624.3 | Semi standard non polar | 33892256 | | N-Acetylmannosamine,3TBDMS,isomer #2 | CC(=O)N[C@@H]1[C@H](O[Si](C)(C)C(C)(C)C)O[C@H](CO[Si](C)(C)C(C)(C)C)[C@@H](O)[C@@H]1O[Si](C)(C)C(C)(C)C | 2664.9 | Semi standard non polar | 33892256 | | N-Acetylmannosamine,3TBDMS,isomer #3 | CC(=O)N([C@@H]1[C@H](O[Si](C)(C)C(C)(C)C)O[C@H](CO[Si](C)(C)C(C)(C)C)[C@@H](O)[C@@H]1O)[Si](C)(C)C(C)(C)C | 2622.2 | Semi standard non polar | 33892256 | | N-Acetylmannosamine,3TBDMS,isomer #4 | CC(=O)N[C@@H]1[C@H](O[Si](C)(C)C(C)(C)C)O[C@H](CO)[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O[Si](C)(C)C(C)(C)C | 2659.1 | Semi standard non polar | 33892256 | | N-Acetylmannosamine,3TBDMS,isomer #5 | CC(=O)N([C@@H]1[C@H](O[Si](C)(C)C(C)(C)C)O[C@H](CO)[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O)[Si](C)(C)C(C)(C)C | 2629.1 | Semi standard non polar | 33892256 | | N-Acetylmannosamine,3TBDMS,isomer #6 | CC(=O)N([C@@H]1[C@H](O[Si](C)(C)C(C)(C)C)O[C@H](CO)[C@@H](O)[C@@H]1O[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2628.6 | Semi standard non polar | 33892256 | | N-Acetylmannosamine,3TBDMS,isomer #7 | CC(=O)N[C@@H]1[C@H](O)O[C@H](CO[Si](C)(C)C(C)(C)C)[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O[Si](C)(C)C(C)(C)C | 2682.8 | Semi standard non polar | 33892256 | | N-Acetylmannosamine,3TBDMS,isomer #8 | CC(=O)N([C@@H]1[C@H](O)O[C@H](CO[Si](C)(C)C(C)(C)C)[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O)[Si](C)(C)C(C)(C)C | 2658.8 | Semi standard non polar | 33892256 | | N-Acetylmannosamine,3TBDMS,isomer #9 | CC(=O)N([C@@H]1[C@H](O)O[C@H](CO[Si](C)(C)C(C)(C)C)[C@@H](O)[C@@H]1O[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2650.9 | Semi standard non polar | 33892256 | | N-Acetylmannosamine,4TBDMS,isomer #1 | CC(=O)N[C@@H]1[C@H](O[Si](C)(C)C(C)(C)C)O[C@H](CO[Si](C)(C)C(C)(C)C)[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O[Si](C)(C)C(C)(C)C | 2872.7 | Semi standard non polar | 33892256 | | N-Acetylmannosamine,4TBDMS,isomer #2 | CC(=O)N([C@@H]1[C@H](O[Si](C)(C)C(C)(C)C)O[C@H](CO[Si](C)(C)C(C)(C)C)[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O)[Si](C)(C)C(C)(C)C | 2860.6 | Semi standard non polar | 33892256 | | N-Acetylmannosamine,4TBDMS,isomer #3 | CC(=O)N([C@@H]1[C@H](O[Si](C)(C)C(C)(C)C)O[C@H](CO[Si](C)(C)C(C)(C)C)[C@@H](O)[C@@H]1O[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2859.4 | Semi standard non polar | 33892256 | | N-Acetylmannosamine,4TBDMS,isomer #4 | CC(=O)N([C@@H]1[C@H](O[Si](C)(C)C(C)(C)C)O[C@H](CO)[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2849.9 | Semi standard non polar | 33892256 | | N-Acetylmannosamine,4TBDMS,isomer #5 | CC(=O)N([C@@H]1[C@H](O)O[C@H](CO[Si](C)(C)C(C)(C)C)[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2868.3 | Semi standard non polar | 33892256 | | N-Acetylmannosamine,5TBDMS,isomer #1 | CC(=O)N([C@@H]1[C@H](O[Si](C)(C)C(C)(C)C)O[C@H](CO[Si](C)(C)C(C)(C)C)[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 3101.4 | Semi standard non polar | 33892256 | | N-Acetylmannosamine,5TBDMS,isomer #1 | CC(=O)N([C@@H]1[C@H](O[Si](C)(C)C(C)(C)C)O[C@H](CO[Si](C)(C)C(C)(C)C)[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 3050.3 | Standard non polar | 33892256 | | N-Acetylmannosamine,5TBDMS,isomer #1 | CC(=O)N([C@@H]1[C@H](O[Si](C)(C)C(C)(C)C)O[C@H](CO[Si](C)(C)C(C)(C)C)[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2705.7 | Standard polar | 33892256 |
|
|---|
| Spectra |
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental GC-MS | GC-MS Spectrum - N-Acetylmannosamine GC-MS (4 TMS) | splash10-00di-1910000000-c54e9f9969c7d963036f | 2018-05-25 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - N-Acetylmannosamine GC-MS (1 MEOX; 5 TMS) | splash10-0pvi-1951000000-56398608d2dd4355e1f6 | 2018-05-25 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - N-Acetylmannosamine GC-MS (1 MEOX; 4 TMS) | splash10-0kw0-3940000000-02993315f1b1a700b7ec | 2018-05-25 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - N-Acetylmannosamine GC-MS (1 MEOX; 5 TMS) | splash10-0pvi-2941000000-c2e3ada4d3e27dc6890a | 2018-05-25 | HMDB team, MONA, MassBank | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - N-Acetylmannosamine GC-MS (Non-derivatized) - 70eV, Positive | splash10-0udl-7920000000-9991e74281c314048f5e | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - N-Acetylmannosamine GC-MS (4 TMS) - 70eV, Positive | splash10-0007-3331900000-c6fab10d67507eecce65 | 2017-10-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - N-Acetylmannosamine GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - N-Acetylmannosamine GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental LC-MS/MS | LC-MS/MS Spectrum - N-Acetylmannosamine Quattro_QQQ 10V, Positive-QTOF (Annotated) | splash10-004r-0920000000-26c87e12e0c8c5f919b7 | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - N-Acetylmannosamine Quattro_QQQ 25V, Positive-QTOF (Annotated) | splash10-001i-9400000000-f0d2f81f4292b627f0ef | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - N-Acetylmannosamine Quattro_QQQ 40V, Positive-QTOF (Annotated) | splash10-001j-9000000000-fcbf9adb619f826e3cd3 | 2012-07-24 | HMDB team, MONA | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - N-Acetylmannosamine 10V, Positive-QTOF | splash10-00di-0390000000-ce54c5d356ec2fc8ab9f | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - N-Acetylmannosamine 20V, Positive-QTOF | splash10-0il0-2950000000-e4e02ffea74d8dfd0810 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - N-Acetylmannosamine 40V, Positive-QTOF | splash10-01ox-9400000000-180503dcc7682bb1753b | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - N-Acetylmannosamine 10V, Negative-QTOF | splash10-00dr-9830000000-a5028d14de09cabbb303 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - N-Acetylmannosamine 20V, Negative-QTOF | splash10-0ac0-9820000000-d3017a9fb13bdc3aa847 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - N-Acetylmannosamine 40V, Negative-QTOF | splash10-0a4l-9200000000-ce24407926ce180ba365 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - N-Acetylmannosamine 10V, Positive-QTOF | splash10-0udi-0390000000-3820a7eec5a8c72f49d9 | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - N-Acetylmannosamine 20V, Positive-QTOF | splash10-0h90-9740000000-db880363194a3979c637 | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - N-Acetylmannosamine 40V, Positive-QTOF | splash10-0229-9200000000-cdcf015aa799dc7d6d8b | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - N-Acetylmannosamine 10V, Negative-QTOF | splash10-0a4i-9500000000-2c2b09cb6bfb29039085 | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - N-Acetylmannosamine 20V, Negative-QTOF | splash10-0abc-9540000000-a1f7d557237a521d306a | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - N-Acetylmannosamine 40V, Negative-QTOF | splash10-0a4i-9000000000-18eabe9e3d3657888360 | 2021-09-22 | Wishart Lab | View Spectrum |
NMR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Experimental 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, H2O, experimental) | 2012-12-05 | Wishart Lab | View Spectrum |
|
|---|
| Biological Properties |
|---|
| Cellular Locations | - Cytoplasm (predicted from logP)
|
|---|
| Biospecimen Locations | |
|---|
| Tissue Locations | - Fibroblasts
- Prostate
- Skeletal Muscle
|
|---|
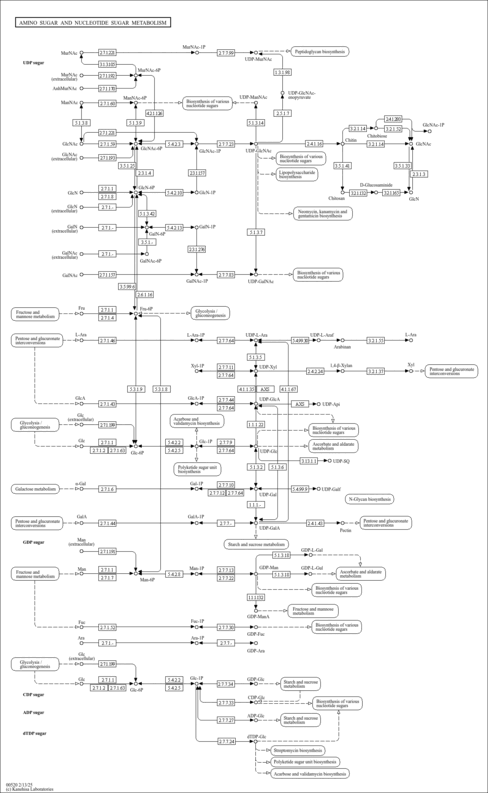
| Pathways | |
|---|
| Normal Concentrations |
|---|
| |
| Feces | Detected but not Quantified | Not Quantified | Infant (0-1 year old) | Both | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Not Specified | Not Specified | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Urine | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details |
|
|---|
| Abnormal Concentrations |
|---|
| |
| Feces | Detected but not Quantified | Not Quantified | Not Specified | Not Specified | Cryptosporidium infection | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Colorectal cancer | | details | | Urine | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Bladder cancer | | details |
|
|---|
| Associated Disorders and Diseases |
|---|
| Disease References | | Colorectal cancer |
|---|
- Goedert JJ, Sampson JN, Moore SC, Xiao Q, Xiong X, Hayes RB, Ahn J, Shi J, Sinha R: Fecal metabolomics: assay performance and association with colorectal cancer. Carcinogenesis. 2014 Sep;35(9):2089-96. doi: 10.1093/carcin/bgu131. Epub 2014 Jul 18. [PubMed:25037050 ]
|
|
|---|
| Associated OMIM IDs | |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| Phenol Explorer Compound ID | Not Available |
|---|
| FooDB ID | FDB028419 |
|---|
| KNApSAcK ID | Not Available |
|---|
| Chemspider ID | 9271300 |
|---|
| KEGG Compound ID | C00645 |
|---|
| BioCyc ID | N-ACETYL-D-MANNOSAMINE |
|---|
| BiGG ID | 35606 |
|---|
| Wikipedia Link | N-acetylmannosamine |
|---|
| METLIN ID | 6024 |
|---|
| PubChem Compound | 11096158 |
|---|
| PDB ID | Not Available |
|---|
| ChEBI ID | 63154 |
|---|
| Food Biomarker Ontology | Not Available |
|---|
| VMH ID | ACMANA |
|---|
| MarkerDB ID | Not Available |
|---|
| Good Scents ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Satoh, Chiyoko; Akio, Kiyomoto. Nitrogen-containing sugars. I. Synthesis of 2-acetamido-2-deoxy-D-mannose from 1-deoxy-1-nitro-D-mannitol pentaacetate. Chemical & Pharmaceutical Bulletin (1964), 12(5), 615-19. |
|---|
| Material Safety Data Sheet (MSDS) | Download (PDF) |
|---|
| General References | - Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, Laxman B, Mehra R, Lonigro RJ, Li Y, Nyati MK, Ahsan A, Kalyana-Sundaram S, Han B, Cao X, Byun J, Omenn GS, Ghosh D, Pennathur S, Alexander DC, Berger A, Shuster JR, Wei JT, Varambally S, Beecher C, Chinnaiyan AM: Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009 Feb 12;457(7231):910-4. doi: 10.1038/nature07762. [PubMed:19212411 ]
- Sala G, Dupre T, Seta N, Codogno P, Ghidoni R: Increased biosynthesis of glycosphingolipids in congenital disorder of glycosylation Ia (CDG-Ia) fibroblasts. Pediatr Res. 2002 Nov;52(5):645-51. [PubMed:12409508 ]
- Salama I, Hinderlich S, Shlomai Z, Eisenberg I, Krause S, Yarema K, Argov Z, Lochmuller H, Reutter W, Dabby R, Sadeh M, Ben-Bassat H, Mitrani-Rosenbaum S: No overall hyposialylation in hereditary inclusion body myopathy myoblasts carrying the homozygous M712T GNE mutation. Biochem Biophys Res Commun. 2005 Mar 4;328(1):221-6. [PubMed:15670773 ]
- Sugawara T, Irie K, Iwasawa H, Yoshikawa T, Okuno S, Watanabe HK, Kato T, Shibukawa M, Ito Y: Synthesis of omega-(methoxycarbonyl)alkyl and 9-(methoxycarbonyl)-3,6-dioxanonyl glycopyranosides for the preparation of carbohydrate-protein conjugates. Carbohydr Res. 1992 Jun 4;230(1):117-49. [PubMed:1511450 ]
- Kamerling JP, Strecker G, Farriaux JP, Dorland L, Haverkamp J, Vliegenthart JF: 2-Acetamidoglucal, a new metabolite isolated from the urine of a patient with sialuria. Biochim Biophys Acta. 1979 Mar 22;583(3):403-8. [PubMed:444571 ]
- von Nicolai H, Esser P, Lauer E: Partial purification and properties of neuraminidase from Bifidobacterium lactentis. Hoppe Seylers Z Physiol Chem. 1981 Feb;362(2):153-62. [PubMed:7216169 ]
- Rodriguez-Aparicio LB, Ferrero MA, Reglero A: N-acetyl-D-neuraminic acid synthesis in Escherichia coli K1 occurs through condensation of N-acetyl-D-mannosamine and pyruvate. Biochem J. 1995 Jun 1;308 ( Pt 2):501-5. [PubMed:7772033 ]
|
|---|