| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Detected and Quantified |
|---|
| Creation Date | 2005-11-16 15:48:42 UTC |
|---|
| Update Date | 2020-09-15 18:18:14 UTC |
|---|
| HMDB ID | HMDB0001319 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Pyridoxine 5'-phosphate |
|---|
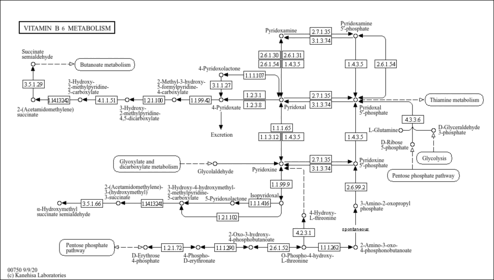
| Description | Pyridoxine 5'-phosphate, also known as pyridoxine-5P or pyridoxine phosphoric acid, belongs to the class of organic compounds known as pyridoxine-5'-phosphates. These are pyridoxines that carry a phosphate group at the 5-position. Pyridoxine 5'-phosphate is a very strong basic compound (based on its pKa). Pyridoxine 5'-phosphate exists in all living species, ranging from bacteria to humans. Within humans, pyridoxine 5'-phosphate participates in a number of enzymatic reactions. In particular, pyridoxine 5'-phosphate can be biosynthesized from pyridoxine through its interaction with the enzyme pyridoxal kinase. In addition, pyridoxine 5'-phosphate can be converted into pyridoxal 5'-phosphate through its interaction with the enzyme pyridoxine-5'-phosphate oxidase. In humans, pyridoxine 5'-phosphate is involved in vitamin B6 metabolism. Outside of the human body, Pyridoxine 5'-phosphate has been detected, but not quantified in, several different foods, such as welsh onions, sweet marjorams, mango, kales, and oil-seed camellia. This could make pyridoxine 5'-phosphate a potential biomarker for the consumption of these foods. |
|---|
| Structure | CC1=NC=C(COP(O)(O)=O)C(CO)=C1O InChI=1S/C8H12NO6P/c1-5-8(11)7(3-10)6(2-9-5)4-15-16(12,13)14/h2,10-11H,3-4H2,1H3,(H2,12,13,14) |
|---|
| Synonyms | | Value | Source |
|---|
| Pyridoxine 5'-(dihydrogen phosphate) | ChEBI | | Pyridoxine 5-phosphate | ChEBI | | Pyridoxine phosphate | ChEBI | | PYRIDOXINE-5'-phosphATE | ChEBI | | Pyridoxol 5'-phosphate | ChEBI | | Pyridoxol 5-phosphate | ChEBI | | Pyridoxyl-5-phosphate | ChEBI | | Pyridoxine 5'-(dihydrogen phosphoric acid) | Generator | | Pyridoxine 5-phosphoric acid | Generator | | Pyridoxine phosphoric acid | Generator | | PYRIDOXINE-5'-phosphoric acid | Generator | | Pyridoxol 5'-phosphoric acid | Generator | | Pyridoxol 5-phosphoric acid | Generator | | Pyridoxyl-5-phosphoric acid | Generator | | Pyridoxine 5'-phosphoric acid | Generator | | 5-Hydroxy-4-(hydroxymethyl)-6-methyl-3-pyridylmethyl dihydrogen phosphate | HMDB | | 5-Hydroxy-6-methyl-3,4-pyridinedimethanol alpha( 3)-(dihydrogen phosphate) | HMDB | | Pyridoxine-5P | HMDB | | Pyridoxine-p | HMDB | | Pyridoxine-phosphate | HMDB | | Pyridoxine 5-phosphate hydrochloride | HMDB | | 5-Phosphopyridoxine | HMDB |
|
|---|
| Chemical Formula | C8H12NO6P |
|---|
| Average Molecular Weight | 249.1577 |
|---|
| Monoisotopic Molecular Weight | 249.040223633 |
|---|
| IUPAC Name | {[5-hydroxy-4-(hydroxymethyl)-6-methylpyridin-3-yl]methoxy}phosphonic acid |
|---|
| Traditional Name | pyridoxine-5'-phosphate |
|---|
| CAS Registry Number | 447-05-2 |
|---|
| SMILES | CC1=NC=C(COP(O)(O)=O)C(CO)=C1O |
|---|
| InChI Identifier | InChI=1S/C8H12NO6P/c1-5-8(11)7(3-10)6(2-9-5)4-15-16(12,13)14/h2,10-11H,3-4H2,1H3,(H2,12,13,14) |
|---|
| InChI Key | WHOMFKWHIQZTHY-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as pyridoxine-5'-phosphates. These are pyridoxines that carry a phosphate group at the 5-position. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pyridines and derivatives |
|---|
| Sub Class | Pyridoxines |
|---|
| Direct Parent | Pyridoxine-5'-phosphates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pyridoxine-5'-phosphate
- Monoalkyl phosphate
- Hydroxypyridine
- Methylpyridine
- Organic phosphoric acid derivative
- Alkyl phosphate
- Phosphoric acid ester
- Heteroaromatic compound
- Azacycle
- Hydrocarbon derivative
- Alcohol
- Aromatic alcohol
- Organic nitrogen compound
- Primary alcohol
- Organooxygen compound
- Organonitrogen compound
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Physiological effect | Not Available |
|---|
| Disposition | |
|---|
| Process | |
|---|
| Role | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Experimental Collision Cross Sections |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Retention Times Underivatized| Chromatographic Method | Retention Time | Reference |
|---|
| Measured using a Waters Acquity ultraperformance liquid chromatography (UPLC) ethylene-bridged hybrid (BEH) C18 column (100 mm × 2.1 mm; 1.7 μmparticle diameter). Predicted by Afia on May 17, 2022. Predicted by Afia on May 17, 2022. | 2.64 minutes | 32390414 | | Predicted by Siyang on May 30, 2022 | 9.6262 minutes | 33406817 | | Predicted by Siyang using ReTip algorithm on June 8, 2022 | 8.12 minutes | 32390414 | | AjsUoB = Accucore 150 Amide HILIC with 10mM Ammonium Formate, 0.1% Formic Acid | 393.6 seconds | 40023050 | | Fem_Long = Waters ACQUITY UPLC HSS T3 C18 with Water:MeOH and 0.1% Formic Acid | 345.9 seconds | 40023050 | | Fem_Lipids = Ascentis Express C18 with (60:40 water:ACN):(90:10 IPA:ACN) and 10mM NH4COOH + 0.1% Formic Acid | 278.3 seconds | 40023050 | | Life_Old = Waters ACQUITY UPLC BEH C18 with Water:(20:80 acetone:ACN) and 0.1% Formic Acid | 59.0 seconds | 40023050 | | Life_New = RP Waters ACQUITY UPLC HSS T3 C18 with Water:(30:70 MeOH:ACN) and 0.1% Formic Acid | 162.3 seconds | 40023050 | | RIKEN = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 39.6 seconds | 40023050 | | Eawag_XBridgeC18 = XBridge C18 3.5u 2.1x50 mm with Water:MeOH and 0.1% Formic Acid | 279.9 seconds | 40023050 | | BfG_NTS_RP1 =Agilent Zorbax Eclipse Plus C18 (2.1 mm x 150 mm, 3.5 um) with Water:ACN and 0.1% Formic Acid | 254.1 seconds | 40023050 | | HILIC_BDD_2 = Merck SeQuant ZIC-HILIC with ACN(0.1% formic acid):water(16 mM ammonium formate) | 815.9 seconds | 40023050 | | UniToyama_Atlantis = RP Waters Atlantis T3 (2.1 x 150 mm, 5 um) with ACN:Water and 0.1% Formic Acid | 589.1 seconds | 40023050 | | BDD_C18 = Hypersil Gold 1.9µm C18 with Water:ACN and 0.1% Formic Acid | 43.7 seconds | 40023050 | | UFZ_Phenomenex = Kinetex Core-Shell C18 2.6 um, 3.0 x 100 mm, Phenomenex with Water:MeOH and 0.1% Formic Acid | 670.5 seconds | 40023050 | | SNU_RIKEN_POS = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 166.7 seconds | 40023050 | | RPMMFDA = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 203.7 seconds | 40023050 | | MTBLS87 = Merck SeQuant ZIC-pHILIC column with ACN:Water and :ammonium carbonate | 647.5 seconds | 40023050 | | KI_GIAR_zic_HILIC_pH2_7 = Merck SeQuant ZIC-HILIC with ACN:Water and 0.1% FA | 570.0 seconds | 40023050 | | Meister zic-pHILIC pH9.3 = Merck SeQuant ZIC-pHILIC column with ACN:Water 5mM NH4Ac pH9.3 and 5mM ammonium acetate in water | 604.1 seconds | 40023050 |
Predicted Kovats Retention IndicesUnderivatizedDerivatized| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| Pyridoxine 5'-phosphate,1TMS,isomer #1 | CC1=NC=C(COP(=O)(O)O)C(CO[Si](C)(C)C)=C1O | 2331.4 | Semi standard non polar | 33892256 | | Pyridoxine 5'-phosphate,1TMS,isomer #2 | CC1=NC=C(COP(=O)(O)O)C(CO)=C1O[Si](C)(C)C | 2250.6 | Semi standard non polar | 33892256 | | Pyridoxine 5'-phosphate,1TMS,isomer #3 | CC1=NC=C(COP(=O)(O)O[Si](C)(C)C)C(CO)=C1O | 2329.5 | Semi standard non polar | 33892256 | | Pyridoxine 5'-phosphate,2TMS,isomer #1 | CC1=NC=C(COP(=O)(O)O)C(CO[Si](C)(C)C)=C1O[Si](C)(C)C | 2262.9 | Semi standard non polar | 33892256 | | Pyridoxine 5'-phosphate,2TMS,isomer #2 | CC1=NC=C(COP(=O)(O)O[Si](C)(C)C)C(CO[Si](C)(C)C)=C1O | 2322.3 | Semi standard non polar | 33892256 | | Pyridoxine 5'-phosphate,2TMS,isomer #3 | CC1=NC=C(COP(=O)(O)O[Si](C)(C)C)C(CO)=C1O[Si](C)(C)C | 2259.0 | Semi standard non polar | 33892256 | | Pyridoxine 5'-phosphate,2TMS,isomer #4 | CC1=NC=C(COP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)C(CO)=C1O | 2298.4 | Semi standard non polar | 33892256 | | Pyridoxine 5'-phosphate,3TMS,isomer #1 | CC1=NC=C(COP(=O)(O)O[Si](C)(C)C)C(CO[Si](C)(C)C)=C1O[Si](C)(C)C | 2316.8 | Semi standard non polar | 33892256 | | Pyridoxine 5'-phosphate,3TMS,isomer #1 | CC1=NC=C(COP(=O)(O)O[Si](C)(C)C)C(CO[Si](C)(C)C)=C1O[Si](C)(C)C | 2244.2 | Standard non polar | 33892256 | | Pyridoxine 5'-phosphate,3TMS,isomer #1 | CC1=NC=C(COP(=O)(O)O[Si](C)(C)C)C(CO[Si](C)(C)C)=C1O[Si](C)(C)C | 2720.5 | Standard polar | 33892256 | | Pyridoxine 5'-phosphate,3TMS,isomer #2 | CC1=NC=C(COP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)C(CO[Si](C)(C)C)=C1O | 2313.4 | Semi standard non polar | 33892256 | | Pyridoxine 5'-phosphate,3TMS,isomer #2 | CC1=NC=C(COP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)C(CO[Si](C)(C)C)=C1O | 2297.8 | Standard non polar | 33892256 | | Pyridoxine 5'-phosphate,3TMS,isomer #2 | CC1=NC=C(COP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)C(CO[Si](C)(C)C)=C1O | 2596.9 | Standard polar | 33892256 | | Pyridoxine 5'-phosphate,3TMS,isomer #3 | CC1=NC=C(COP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)C(CO)=C1O[Si](C)(C)C | 2243.6 | Semi standard non polar | 33892256 | | Pyridoxine 5'-phosphate,3TMS,isomer #3 | CC1=NC=C(COP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)C(CO)=C1O[Si](C)(C)C | 2269.7 | Standard non polar | 33892256 | | Pyridoxine 5'-phosphate,3TMS,isomer #3 | CC1=NC=C(COP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)C(CO)=C1O[Si](C)(C)C | 2508.2 | Standard polar | 33892256 | | Pyridoxine 5'-phosphate,4TMS,isomer #1 | CC1=NC=C(COP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)C(CO[Si](C)(C)C)=C1O[Si](C)(C)C | 2327.4 | Semi standard non polar | 33892256 | | Pyridoxine 5'-phosphate,4TMS,isomer #1 | CC1=NC=C(COP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)C(CO[Si](C)(C)C)=C1O[Si](C)(C)C | 2278.9 | Standard non polar | 33892256 | | Pyridoxine 5'-phosphate,4TMS,isomer #1 | CC1=NC=C(COP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)C(CO[Si](C)(C)C)=C1O[Si](C)(C)C | 2457.1 | Standard polar | 33892256 | | Pyridoxine 5'-phosphate,1TBDMS,isomer #1 | CC1=NC=C(COP(=O)(O)O)C(CO[Si](C)(C)C(C)(C)C)=C1O | 2568.8 | Semi standard non polar | 33892256 | | Pyridoxine 5'-phosphate,1TBDMS,isomer #2 | CC1=NC=C(COP(=O)(O)O)C(CO)=C1O[Si](C)(C)C(C)(C)C | 2520.0 | Semi standard non polar | 33892256 | | Pyridoxine 5'-phosphate,1TBDMS,isomer #3 | CC1=NC=C(COP(=O)(O)O[Si](C)(C)C(C)(C)C)C(CO)=C1O | 2577.1 | Semi standard non polar | 33892256 | | Pyridoxine 5'-phosphate,2TBDMS,isomer #1 | CC1=NC=C(COP(=O)(O)O)C(CO[Si](C)(C)C(C)(C)C)=C1O[Si](C)(C)C(C)(C)C | 2726.6 | Semi standard non polar | 33892256 | | Pyridoxine 5'-phosphate,2TBDMS,isomer #2 | CC1=NC=C(COP(=O)(O)O[Si](C)(C)C(C)(C)C)C(CO[Si](C)(C)C(C)(C)C)=C1O | 2762.4 | Semi standard non polar | 33892256 | | Pyridoxine 5'-phosphate,2TBDMS,isomer #3 | CC1=NC=C(COP(=O)(O)O[Si](C)(C)C(C)(C)C)C(CO)=C1O[Si](C)(C)C(C)(C)C | 2734.4 | Semi standard non polar | 33892256 | | Pyridoxine 5'-phosphate,2TBDMS,isomer #4 | CC1=NC=C(COP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)C(CO)=C1O | 2772.7 | Semi standard non polar | 33892256 | | Pyridoxine 5'-phosphate,3TBDMS,isomer #1 | CC1=NC=C(COP(=O)(O)O[Si](C)(C)C(C)(C)C)C(CO[Si](C)(C)C(C)(C)C)=C1O[Si](C)(C)C(C)(C)C | 2982.1 | Semi standard non polar | 33892256 | | Pyridoxine 5'-phosphate,3TBDMS,isomer #1 | CC1=NC=C(COP(=O)(O)O[Si](C)(C)C(C)(C)C)C(CO[Si](C)(C)C(C)(C)C)=C1O[Si](C)(C)C(C)(C)C | 2798.8 | Standard non polar | 33892256 | | Pyridoxine 5'-phosphate,3TBDMS,isomer #1 | CC1=NC=C(COP(=O)(O)O[Si](C)(C)C(C)(C)C)C(CO[Si](C)(C)C(C)(C)C)=C1O[Si](C)(C)C(C)(C)C | 2985.4 | Standard polar | 33892256 | | Pyridoxine 5'-phosphate,3TBDMS,isomer #2 | CC1=NC=C(COP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)C(CO[Si](C)(C)C(C)(C)C)=C1O | 2976.9 | Semi standard non polar | 33892256 | | Pyridoxine 5'-phosphate,3TBDMS,isomer #2 | CC1=NC=C(COP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)C(CO[Si](C)(C)C(C)(C)C)=C1O | 2831.4 | Standard non polar | 33892256 | | Pyridoxine 5'-phosphate,3TBDMS,isomer #2 | CC1=NC=C(COP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)C(CO[Si](C)(C)C(C)(C)C)=C1O | 2911.1 | Standard polar | 33892256 | | Pyridoxine 5'-phosphate,3TBDMS,isomer #3 | CC1=NC=C(COP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)C(CO)=C1O[Si](C)(C)C(C)(C)C | 2927.8 | Semi standard non polar | 33892256 | | Pyridoxine 5'-phosphate,3TBDMS,isomer #3 | CC1=NC=C(COP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)C(CO)=C1O[Si](C)(C)C(C)(C)C | 2764.9 | Standard non polar | 33892256 | | Pyridoxine 5'-phosphate,3TBDMS,isomer #3 | CC1=NC=C(COP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)C(CO)=C1O[Si](C)(C)C(C)(C)C | 2821.3 | Standard polar | 33892256 | | Pyridoxine 5'-phosphate,4TBDMS,isomer #1 | CC1=NC=C(COP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)C(CO[Si](C)(C)C(C)(C)C)=C1O[Si](C)(C)C(C)(C)C | 3186.1 | Semi standard non polar | 33892256 | | Pyridoxine 5'-phosphate,4TBDMS,isomer #1 | CC1=NC=C(COP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)C(CO[Si](C)(C)C(C)(C)C)=C1O[Si](C)(C)C(C)(C)C | 2910.0 | Standard non polar | 33892256 | | Pyridoxine 5'-phosphate,4TBDMS,isomer #1 | CC1=NC=C(COP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)C(CO[Si](C)(C)C(C)(C)C)=C1O[Si](C)(C)C(C)(C)C | 2847.5 | Standard polar | 33892256 |
|
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - Pyridoxine 5'-phosphate GC-MS (Non-derivatized) - 70eV, Positive | splash10-0002-9530000000-1e967afad92165e82bee | 2016-09-22 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Pyridoxine 5'-phosphate GC-MS (2 TMS) - 70eV, Positive | splash10-00di-9226000000-49f5293644ca681d401b | 2017-10-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Pyridoxine 5'-phosphate GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Pyridoxine 5'-phosphate GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Pyridoxine 5'-phosphate 10V, Positive-QTOF | splash10-0ue9-0590000000-68640055ed308d852fd0 | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Pyridoxine 5'-phosphate 20V, Positive-QTOF | splash10-0f89-0900000000-1450b9f084acf6421d59 | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Pyridoxine 5'-phosphate 40V, Positive-QTOF | splash10-001i-4900000000-8fd6492e6f2a6bea9879 | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Pyridoxine 5'-phosphate 10V, Negative-QTOF | splash10-0002-8090000000-65f73f2c7a460a3fca23 | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Pyridoxine 5'-phosphate 20V, Negative-QTOF | splash10-004i-9010000000-5b0859a6a40a458fcc9f | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Pyridoxine 5'-phosphate 40V, Negative-QTOF | splash10-004i-9000000000-a6fead236e12d88986b8 | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Pyridoxine 5'-phosphate 10V, Positive-QTOF | splash10-0udi-0290000000-da88c1789911b79c0362 | 2021-09-23 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Pyridoxine 5'-phosphate 20V, Positive-QTOF | splash10-0ff0-0910000000-366dc9237952c888f4b8 | 2021-09-23 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Pyridoxine 5'-phosphate 40V, Positive-QTOF | splash10-001i-2900000000-cd37cb30cd544c5f866a | 2021-09-23 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Pyridoxine 5'-phosphate 10V, Negative-QTOF | splash10-002b-9010000000-64a9d47104102ec4486f | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Pyridoxine 5'-phosphate 20V, Negative-QTOF | splash10-004i-9000000000-ef724d5177083af378f0 | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Pyridoxine 5'-phosphate 40V, Negative-QTOF | splash10-004i-9000000000-a5e502a2627af2048a1f | 2021-09-24 | Wishart Lab | View Spectrum |
NMR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum |
|
|---|