| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Detected and Quantified |
|---|
| Creation Date | 2005-11-16 15:48:42 UTC |
|---|
| Update Date | 2021-09-14 14:57:20 UTC |
|---|
| HMDB ID | HMDB0001487 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | NADH |
|---|
| Description | Nicotinamide adenine dinucleotide (NAD) is a coenzyme central to metabolism. Found in all living cells, NAD is called a dinucleotide because it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine nucleobase and the other nicotinamide. NAD exists in two forms: an oxidized and reduced form, abbreviated as NAD+ and NADH (H for hydrogen) respectively. NADH is the reduced form of NAD+, and NAD+ is the oxidized form of NADH. NAD (or nicotinamide adenine dinucleotide) is used extensively in glycolysis and the citric acid cycle of cellular respiration. The reducing potential stored in NADH can be either converted into ATP through the electron transport chain or used for anabolic metabolism. ATP "energy" is necessary for an organism to live. Green plants obtain ATP through photosynthesis, while other organisms obtain it via cellular respiration. NAD is a coenzyme composed of ribosylnicotinamide 5'-diphosphate coupled to adenosine 5'-phosphate by a pyrophosphate linkage. It is found widely in nature and is involved in numerous enzymatic reactions in which it serves as an electron carrier by being alternately oxidized (NAD+) and reduced (NADH). NADP is formed through the addition of a phosphate group to the 2' position of the adenosyl nucleotide through an ester linkage. |
|---|
| Structure | NC(=O)C1=CN(C=CC1)[C@@H]1O[C@H](COP(O)(=O)OP(O)(=O)OC[C@H]2O[C@H]([C@H](O)[C@@H]2O)N2C=NC3=C2N=CN=C3N)[C@@H](O)[C@H]1O InChI=1S/C21H29N7O14P2/c22-17-12-19(25-7-24-17)28(8-26-12)21-16(32)14(30)11(41-21)6-39-44(36,37)42-43(34,35)38-5-10-13(29)15(31)20(40-10)27-3-1-2-9(4-27)18(23)33/h1,3-4,7-8,10-11,13-16,20-21,29-32H,2,5-6H2,(H2,23,33)(H,34,35)(H,36,37)(H2,22,24,25)/t10-,11-,13-,14-,15-,16-,20-,21-/m1/s1 |
|---|
| Synonyms | | Value | Source |
|---|
| 1,4-DIHYDRONICOTINAMIDE adenine dinucleotide | ChEBI | | DPNH | ChEBI | | Nicotinamide adenine dinucleotide (reduced) | ChEBI | | Reduced nicotinamide adenine dinucleotide | ChEBI | | b-DPNH | HMDB | | b-NADH | HMDB | | beta-DPNH | HMDB | | beta-NADH | HMDB | | Dihydrocodehydrogenase I | HMDB | | Dihydrocozymase | HMDB | | Dihydronicotinamide adenine dinucleotide | HMDB | | Dihydronicotinamide mononucleotide | HMDB | | ENADA | HMDB | | NADH2 | HMDB | | Reduced codehydrogenase I | HMDB | | Reduced diphosphopyridine nucleotide | HMDB | | Reduced nicotinamide adenine diphosphate | HMDB | | Reduced nicotinamide-adenine dinucleotide | HMDB | | Nadide | HMDB | | Coenzyme I | HMDB | | DPN | HMDB | | Diphosphopyridine nucleotide | HMDB | | Nicotinamide adenine dinucleotide | HMDB | | Nicotinamide-adenine dinucleotide | HMDB | | NAD | HMDB | | Nucleotide, diphosphopyridine | HMDB | | Adenine dinucleotide, dihydronicotinamide | HMDB | | Dinucleotide, dihydronicotinamide adenine | HMDB | | Dinucleotide, nicotinamide-adenine | HMDB |
|
|---|
| Chemical Formula | C21H29N7O14P2 |
|---|
| Average Molecular Weight | 665.441 |
|---|
| Monoisotopic Molecular Weight | 665.124771695 |
|---|
| IUPAC Name | [({[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy]({[(2R,3S,4R,5R)-5-(3-carbamoyl-1,4-dihydropyridin-1-yl)-3,4-dihydroxyoxolan-2-yl]methoxy})phosphinic acid |
|---|
| Traditional Name | NADH |
|---|
| CAS Registry Number | 58-68-4 |
|---|
| SMILES | NC(=O)C1=CN(C=CC1)[C@@H]1O[C@H](COP(O)(=O)OP(O)(=O)OC[C@H]2O[C@H]([C@H](O)[C@@H]2O)N2C=NC3=C2N=CN=C3N)[C@@H](O)[C@H]1O |
|---|
| InChI Identifier | InChI=1S/C21H29N7O14P2/c22-17-12-19(25-7-24-17)28(8-26-12)21-16(32)14(30)11(41-21)6-39-44(36,37)42-43(34,35)38-5-10-13(29)15(31)20(40-10)27-3-1-2-9(4-27)18(23)33/h1,3-4,7-8,10-11,13-16,20-21,29-32H,2,5-6H2,(H2,23,33)(H,34,35)(H,36,37)(H2,22,24,25)/t10-,11-,13-,14-,15-,16-,20-,21-/m1/s1 |
|---|
| InChI Key | BOPGDPNILDQYTO-NNYOXOHSSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as (5'->5')-dinucleotides. These are dinucleotides where the two bases are connected via a (5'->5')-phosphodiester linkage. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Nucleosides, nucleotides, and analogues |
|---|
| Class | (5'->5')-dinucleotides |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | (5'->5')-dinucleotides |
|---|
| Alternative Parents | |
|---|
| Substituents | - (5'->5')-dinucleotide
- Purine nucleotide sugar
- Purine ribonucleoside diphosphate
- Purine ribonucleoside monophosphate
- Nicotinamide-nucleotide
- Pentose phosphate
- Pentose-5-phosphate
- Glycosyl compound
- N-glycosyl compound
- 6-aminopurine
- Monosaccharide phosphate
- N-substituted nicotinamide
- Organic pyrophosphate
- Imidazopyrimidine
- Purine
- Monoalkyl phosphate
- Dihydropyridine
- Aminopyrimidine
- Pyrimidine
- Imidolactam
- Monosaccharide
- N-substituted imidazole
- Alkyl phosphate
- Phosphoric acid ester
- Hydropyridine
- Organic phosphoric acid derivative
- Heteroaromatic compound
- Tetrahydrofuran
- Imidazole
- Vinylogous amide
- Azole
- Amino acid or derivatives
- Primary carboxylic acid amide
- Secondary alcohol
- Carboxamide group
- Organoheterocyclic compound
- Enamine
- Azacycle
- Oxacycle
- Carboxylic acid derivative
- Hydrocarbon derivative
- Alcohol
- Organic nitrogen compound
- Organonitrogen compound
- Organooxygen compound
- Carbonyl group
- Organic oxygen compound
- Organopnictogen compound
- Primary amine
- Amine
- Organic oxide
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Not Available | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | 140.0 - 142.0 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Experimental Collision Cross Sections| Adduct Type | Data Source | CCS Value (Å2) | Reference |
|---|
| [M-H]- | Astarita_neg | 224.3 | 30932474 |
|
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Retention Times Underivatized| Chromatographic Method | Retention Time | Reference |
|---|
| Measured using a Waters Acquity ultraperformance liquid chromatography (UPLC) ethylene-bridged hybrid (BEH) C18 column (100 mm × 2.1 mm; 1.7 μmparticle diameter). Predicted by Afia on May 17, 2022. Predicted by Afia on May 17, 2022. | 2.52 minutes | 32390414 | | Predicted by Siyang on May 30, 2022 | 10.7609 minutes | 33406817 | | Predicted by Siyang using ReTip algorithm on June 8, 2022 | 9.1 minutes | 32390414 | | AjsUoB = Accucore 150 Amide HILIC with 10mM Ammonium Formate, 0.1% Formic Acid | 491.1 seconds | 40023050 | | Fem_Long = Waters ACQUITY UPLC HSS T3 C18 with Water:MeOH and 0.1% Formic Acid | 1090.0 seconds | 40023050 | | Fem_Lipids = Ascentis Express C18 with (60:40 water:ACN):(90:10 IPA:ACN) and 10mM NH4COOH + 0.1% Formic Acid | 213.1 seconds | 40023050 | | Life_Old = Waters ACQUITY UPLC BEH C18 with Water:(20:80 acetone:ACN) and 0.1% Formic Acid | 56.5 seconds | 40023050 | | Life_New = RP Waters ACQUITY UPLC HSS T3 C18 with Water:(30:70 MeOH:ACN) and 0.1% Formic Acid | 172.7 seconds | 40023050 | | RIKEN = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 71.8 seconds | 40023050 | | Eawag_XBridgeC18 = XBridge C18 3.5u 2.1x50 mm with Water:MeOH and 0.1% Formic Acid | 345.6 seconds | 40023050 | | BfG_NTS_RP1 =Agilent Zorbax Eclipse Plus C18 (2.1 mm x 150 mm, 3.5 um) with Water:ACN and 0.1% Formic Acid | 338.6 seconds | 40023050 | | HILIC_BDD_2 = Merck SeQuant ZIC-HILIC with ACN(0.1% formic acid):water(16 mM ammonium formate) | 839.1 seconds | 40023050 | | UniToyama_Atlantis = RP Waters Atlantis T3 (2.1 x 150 mm, 5 um) with ACN:Water and 0.1% Formic Acid | 645.7 seconds | 40023050 | | BDD_C18 = Hypersil Gold 1.9µm C18 with Water:ACN and 0.1% Formic Acid | 168.1 seconds | 40023050 | | UFZ_Phenomenex = Kinetex Core-Shell C18 2.6 um, 3.0 x 100 mm, Phenomenex with Water:MeOH and 0.1% Formic Acid | 840.8 seconds | 40023050 | | SNU_RIKEN_POS = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 230.1 seconds | 40023050 | | RPMMFDA = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 290.4 seconds | 40023050 | | MTBLS87 = Merck SeQuant ZIC-pHILIC column with ACN:Water and :ammonium carbonate | 587.9 seconds | 40023050 | | KI_GIAR_zic_HILIC_pH2_7 = Merck SeQuant ZIC-HILIC with ACN:Water and 0.1% FA | 429.3 seconds | 40023050 | | Meister zic-pHILIC pH9.3 = Merck SeQuant ZIC-pHILIC column with ACN:Water 5mM NH4Ac pH9.3 and 5mM ammonium acetate in water | 608.4 seconds | 40023050 |
Predicted Kovats Retention IndicesUnderivatized |
|---|
| Spectra |
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - NADH GC-MS (Non-derivatized) - 70eV, Positive | splash10-002b-1301902000-1a1639ad87019605438c | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADH GC-MS (TMS_1_1) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADH GC-MS (TMS_1_2) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADH GC-MS (TMS_1_3) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADH GC-MS (TMS_1_4) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADH GC-MS (TMS_1_5) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADH GC-MS (TMS_1_6) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADH GC-MS (TMS_1_7) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADH GC-MS (TMS_1_8) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADH GC-MS (TMS_2_1) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADH GC-MS (TMS_2_2) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADH GC-MS (TMS_2_3) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADH GC-MS (TMS_2_4) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADH GC-MS (TMS_2_5) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADH GC-MS (TMS_2_6) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADH GC-MS (TMS_2_7) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADH GC-MS (TMS_2_8) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADH GC-MS (TMS_2_9) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADH GC-MS (TMS_2_10) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADH GC-MS (TMS_2_11) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADH GC-MS (TMS_2_12) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADH GC-MS (TMS_2_13) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADH GC-MS (TMS_2_14) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADH GC-MS (TMS_2_15) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADH GC-MS (TMS_2_16) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental LC-MS/MS | LC-MS/MS Spectrum - NADH Quattro_QQQ 10V, Positive-QTOF (Annotated) | splash10-00n0-0210192000-bf07b6b154c5778067ce | 2018-05-25 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - NADH Quattro_QQQ 25V, Positive-QTOF (Annotated) | splash10-0udi-0150291000-84ef746651797f0679a5 | 2018-05-25 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - NADH Quattro_QQQ 40V, Positive-QTOF (Annotated) | splash10-004i-0970000000-0688003193d7fc461235 | 2018-05-25 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - NADH LC-ESI-ITFT 35V, negative-QTOF | splash10-00di-0190000000-775fe0fdd09a45ed6a96 | 2020-07-21 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - NADH LC-ESI-ITFT 35V, negative-QTOF | splash10-052b-0019600000-3247f3ea96ec22c0e190 | 2020-07-21 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - NADH LC-ESI-ITFT 35V, negative-QTOF | splash10-052b-0019600000-c62f2c56a1ce8a2874fb | 2020-07-21 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - NADH LC-ESI-QTOF 32V, negative-QTOF | splash10-03di-3212549000-5bb7a136d3e7ca7d8edd | 2020-07-21 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - NADH LC-ESI-QTOF 30V, negative-QTOF | splash10-00ea-1219600000-1e722b38e19c9839c336 | 2020-07-21 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - NADH 30V, Positive-QTOF | splash10-00ea-1219600000-25943c109cf639eb967c | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - NADH 30V, Positive-QTOF | splash10-05dj-5449401000-5b1b9b660a66672f95af | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - NADH 20V, Negative-QTOF | splash10-03di-0000009000-c3a8f40f5761a0b33639 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - NADH 40V, Negative-QTOF | splash10-054k-6339301000-c1dfbc12dc893f53f0a4 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - NADH 10V, Negative-QTOF | splash10-03di-0000009000-ced4da70fe9076bb4c29 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - NADH 30V, Negative-QTOF | splash10-05dj-5449401000-1c9466169a2e22067bd9 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - NADH 10V, Positive-QTOF | splash10-00kb-0000009000-f46730c3cbc0e239c819 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - NADH 40V, Positive-QTOF | splash10-0udi-1329230000-4e5a5ac7f7ccdfcf5798 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - NADH 30V, Negative-QTOF | splash10-00ea-1219600000-1e722b38e19c9839c336 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - NADH 20V, Positive-QTOF | splash10-0002-0002049000-452a7a02c7cdd0a7db02 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - NADH 10V, Positive-QTOF | splash10-000i-0931104000-bf6579d19ee9bc297e13 | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - NADH 20V, Positive-QTOF | splash10-000i-0901000000-92672b1d4b96838f8652 | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - NADH 40V, Positive-QTOF | splash10-000i-1900000000-ef56d203da65089e145f | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - NADH 10V, Negative-QTOF | splash10-03e9-1900207000-f248eb28a7283de7118b | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - NADH 20V, Negative-QTOF | splash10-001i-1900100000-efadf1d6c54e9e86671d | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - NADH 40V, Negative-QTOF | splash10-0a7i-3900000000-f258fc2bf1d8e54ac6da | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - NADH 10V, Negative-QTOF | splash10-03di-0000009000-7483a50cefc91ed6c523 | 2021-09-22 | Wishart Lab | View Spectrum |
NMR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Experimental 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, H2O, experimental) | 2018-05-25 | Wishart Lab | View Spectrum |
|
|---|
| Biological Properties |
|---|
| Cellular Locations | - Mitochondria
- Endoplasmic reticulum
- Peroxisome
|
|---|
| Biospecimen Locations | |
|---|
| Tissue Locations | - Bladder
- Brain
- Fibroblasts
- Platelet
- Skeletal Muscle
|
|---|
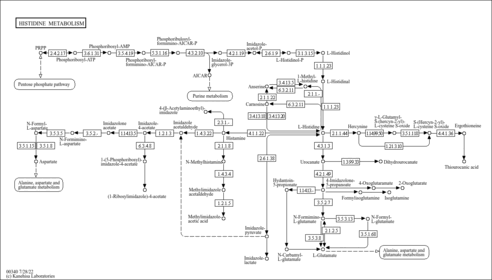
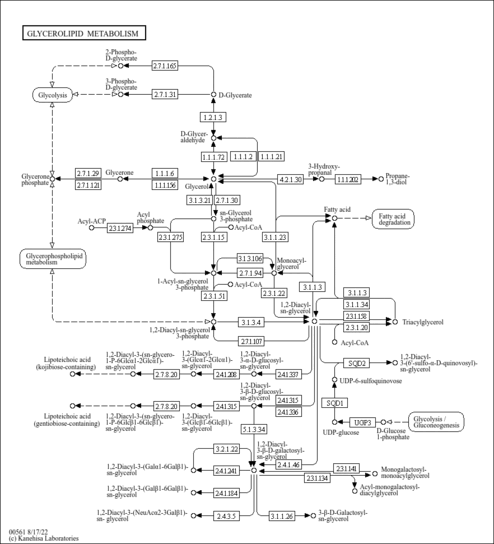
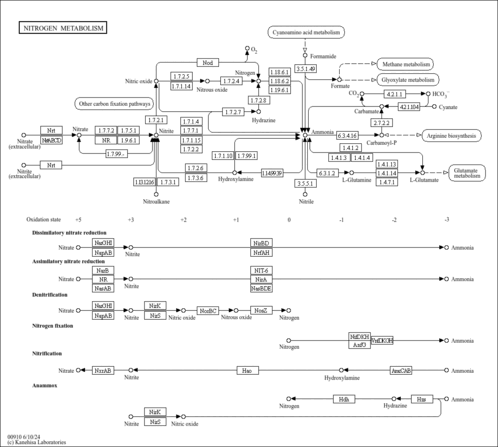
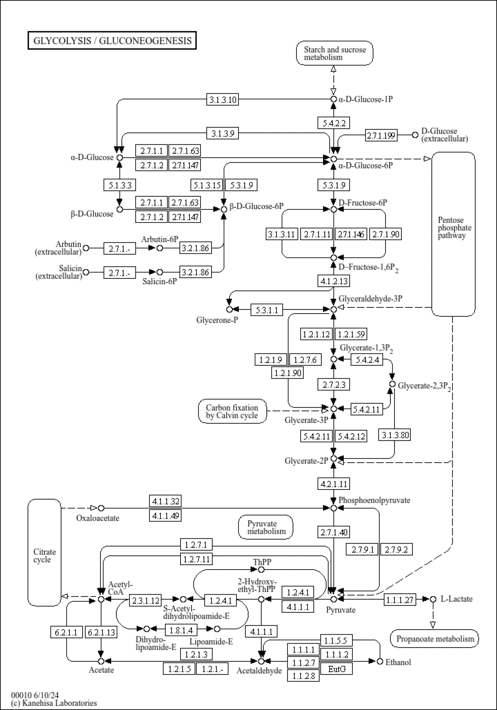
| Pathways | |
|---|
| Normal Concentrations |
|---|
| |
| Blood | Detected and Quantified | 22.0 (14.0-40.0) uM | Adult (>18 years old) | Both | Normal | | details |
|
|---|
| Abnormal Concentrations |
|---|
| |
| Blood | Detected and Quantified | 0.0081 uM | Infant (0-1 year old) | Female | Nicotinamide Adenine Dinucleotide Deficiency | | details |
|
|---|
| Associated Disorders and Diseases |
|---|
| Disease References | | Nicotinamide Adenine Dinucleotide Deficiency |
|---|
- Shi H, Enriquez A, Rapadas M, Martin EMMA, Wang R, Moreau J, Lim CK, Szot JO, Ip E, Hughes JN, Sugimoto K, Humphreys DT, McInerney-Leo AM, Leo PJ, Maghzal GJ, Halliday J, Smith J, Colley A, Mark PR, Collins F, Sillence DO, Winlaw DS, Ho JWK, Guillemin GJ, Brown MA, Kikuchi K, Thomas PQ, Stocker R, Giannoulatou E, Chapman G, Duncan EL, Sparrow DB, Dunwoodie SL: NAD Deficiency, Congenital Malformations, and Niacin Supplementation. N Engl J Med. 2017 Aug 10;377(6):544-552. doi: 10.1056/NEJMoa1616361. [PubMed:28792876 ]
|
|
|---|
| Associated OMIM IDs | None |
|---|
| External Links |
|---|
| DrugBank ID | DB00157 |
|---|
| Phenol Explorer Compound ID | Not Available |
|---|
| FooDB ID | FDB022649 |
|---|
| KNApSAcK ID | C00019343 |
|---|
| Chemspider ID | 903 |
|---|
| KEGG Compound ID | C00004 |
|---|
| BioCyc ID | NADH |
|---|
| BiGG ID | 33484 |
|---|
| Wikipedia Link | Nicotinamide_adenine_dinucleotide |
|---|
| METLIN ID | 3687 |
|---|
| PubChem Compound | 439153 |
|---|
| PDB ID | Not Available |
|---|
| ChEBI ID | 16908 |
|---|
| Food Biomarker Ontology | Not Available |
|---|
| VMH ID | HC02112 |
|---|
| MarkerDB ID | Not Available |
|---|
| Good Scents ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Marek, Miroslav; Vrbova, Eva; Horakova, Irena; Musil, Petr; Kefurt, Karel. NADH manufacture with immobilized Candida formate dehydrogenase. Czech. (1992), 4 pp. |
|---|
| Material Safety Data Sheet (MSDS) | Download (PDF) |
|---|
| General References | - Krotz F, Sohn HY, Gloe T, Zahler S, Riexinger T, Schiele TM, Becker BF, Theisen K, Klauss V, Pohl U: NAD(P)H oxidase-dependent platelet superoxide anion release increases platelet recruitment. Blood. 2002 Aug 1;100(3):917-24. [PubMed:12130503 ]
- Yamamoto T, Moriwaki Y, Takahashi S, Suda M, Higashino K: Ethanol as a xanthine dehydrogenase inhibitor. Metabolism. 1995 Jun;44(6):779-85. [PubMed:7783663 ]
- Nadlinger K, Westerthaler W, Storga-Tomic D, Birkmayer JG: Extracellular metabolisation of NADH by blood cells correlates with intracellular ATP levels. Biochim Biophys Acta. 2002 Nov 14;1573(2):177-82. [PubMed:12399028 ]
- Saada A, Bar-Meir M, Belaiche C, Miller C, Elpeleg O: Evaluation of enzymatic assays and compounds affecting ATP production in mitochondrial respiratory chain complex I deficiency. Anal Biochem. 2004 Dec 1;335(1):66-72. [PubMed:15519572 ]
- Heiman-Patterson TD, Argov Z, Chavin JM, Kalman B, Alder H, DiMauro S, Bank W, Tahmoush AJ: Biochemical and genetic studies in a family with mitochondrial myopathy. Muscle Nerve. 1997 Oct;20(10):1219-24. [PubMed:9324076 ]
- Mintun MA, Vlassenko AG, Rundle MM, Raichle ME: Increased lactate/pyruvate ratio augments blood flow in physiologically activated human brain. Proc Natl Acad Sci U S A. 2004 Jan 13;101(2):659-64. Epub 2004 Jan 2. [PubMed:14704276 ]
- Yeo SF, Zhang Y, Schafer D, Campbell S, Wong B: A rapid, automated enzymatic fluorometric assay for determination of D-arabinitol in serum. J Clin Microbiol. 2000 Apr;38(4):1439-43. [PubMed:10747122 ]
- Uppal A, Ghosh N, Datta A, Gupta PK: Fluorimetric estimation of the concentration of NADH from human blood samples. Biotechnol Appl Biochem. 2005 Feb;41(Pt 1):43-7. [PubMed:15035655 ]
- Yamamoto T, Moriwaki Y, Takahashi S, Suda M, Higashino K: Xylitol-induced increase in the concentration of oxypurines and its mechanism. Int J Clin Pharmacol Ther. 1995 Jun;33(6):360-5. [PubMed:7582389 ]
- Helge JW, Fraser AM, Kriketos AD, Jenkins AB, Calvert GD, Ayre KJ, Storlien LH: Interrelationships between muscle fibre type, substrate oxidation and body fat. Int J Obes Relat Metab Disord. 1999 Sep;23(9):986-91. [PubMed:10490806 ]
- Fulco M, Schiltz RL, Iezzi S, King MT, Zhao P, Kashiwaya Y, Hoffman E, Veech RL, Sartorelli V: Sir2 regulates skeletal muscle differentiation as a potential sensor of the redox state. Mol Cell. 2003 Jul;12(1):51-62. [PubMed:12887892 ]
- Li D, Gan Y, Wientjes MG, Badalament RA, Au JL: Distribution of DT-diaphorase and reduced nicotinamide adenine dinucleotide phosphate: cytochrome p450 oxidoreductase in bladder tissues and tumors. J Urol. 2001 Dec;166(6):2500-5. [PubMed:11696818 ]
- Desir G, Bratusch-Marrain P, DeFronzo RA: Effect of hyperketonemia on renal ammonia excretion in man. Metabolism. 1986 Aug;35(8):736-43. [PubMed:3736414 ]
- Odland LM, Heigenhauser GJ, Spriet LL: Effects of high fat provision on muscle PDH activation and malonyl-CoA content in moderate exercise. J Appl Physiol (1985). 2000 Dec;89(6):2352-8. [PubMed:11090589 ]
- Rani K, Garg P, Pundir CS: Measurement of bile acid in serum and bile with arylamine-glass-bound 3alpha-hydroxysteroid dehydrogenase and diaphorase. Anal Biochem. 2004 Sep 1;332(1):32-7. [PubMed:15301946 ]
- Nomura H, Koike F, Tsuruta Y, Iwaki A, Iwaki T: Autopsy case of autosomal recessive hereditary spastic paraplegia with reference to the muscular pathology. Neuropathology. 2001 Sep;21(3):212-7. [PubMed:11666018 ]
- Orallo F, Alvarez E, Camina M, Leiro JM, Gomez E, Fernandez P: The possible implication of trans-Resveratrol in the cardioprotective effects of long-term moderate wine consumption. Mol Pharmacol. 2002 Feb;61(2):294-302. [PubMed:11809853 ]
- Jawed S, Stevens CR, Harrison R, Blake DR: Elevated circulating plasma NADH oxidising activity of xanthine oxidoreductase in plasma. Biochem Soc Trans. 1997 Aug;25(3):531S. [PubMed:9388747 ]
- Harbord MG, Hwang PA, Robinson BH, Becker LE, Hunjan A, Murphy EG: Infant-onset progressive myoclonus epilepsy. J Child Neurol. 1991 Apr;6(2):134-42. [PubMed:1904460 ]
- Mayevsky A, Meilin S, Manor T, Ornstein E, Zarchin N, Sonn J: Multiparametric monitoring of brain oxygen balance under experimental and clinical conditions. Neurol Res. 1998;20 Suppl 1:S76-80. [PubMed:9584930 ]
- Biellmann JF, Lapinte C, Haid E, Weimann G: Structure of lactate dehydrogenase inhibitor generated from coenzyme. Biochemistry. 1979 Apr 3;18(7):1212-7. [PubMed:218616 ]
- Lin SJ, Guarente L: Nicotinamide adenine dinucleotide, a metabolic regulator of transcription, longevity and disease. Curr Opin Cell Biol. 2003 Apr;15(2):241-6. [PubMed:12648681 ]
- Belenky P, Bogan KL, Brenner C: NAD+ metabolism in health and disease. Trends Biochem Sci. 2007 Jan;32(1):12-9. Epub 2006 Dec 11. [PubMed:17161604 ]
- Pollak N, Dolle C, Ziegler M: The power to reduce: pyridine nucleotides--small molecules with a multitude of functions. Biochem J. 2007 Mar 1;402(2):205-18. [PubMed:17295611 ]
- Khan JA, Forouhar F, Tao X, Tong L: Nicotinamide adenine dinucleotide metabolism as an attractive target for drug discovery. Expert Opin Ther Targets. 2007 May;11(5):695-705. [PubMed:17465726 ]
- Brunk E, Sahoo S, Zielinski DC, Altunkaya A, Drager A, Mih N, Gatto F, Nilsson A, Preciat Gonzalez GA, Aurich MK, Prlic A, Sastry A, Danielsdottir AD, Heinken A, Noronha A, Rose PW, Burley SK, Fleming RMT, Nielsen J, Thiele I, Palsson BO: Recon3D enables a three-dimensional view of gene variation in human metabolism. Nat Biotechnol. 2018 Mar;36(3):272-281. doi: 10.1038/nbt.4072. Epub 2018 Feb 19. [PubMed:29457794 ]
- WholeHealthMD [Link]
|
|---|