| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Expected but not Quantified |
|---|
| Creation Date | 2005-11-16 15:48:42 UTC |
|---|
| Update Date | 2023-02-21 17:15:45 UTC |
|---|
| HMDB ID | HMDB0001494 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Acetylphosphate |
|---|
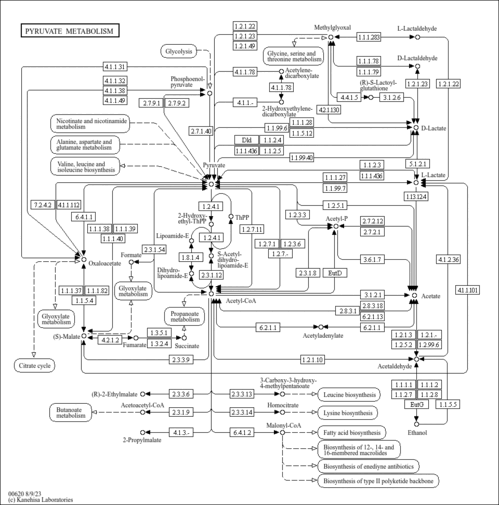
| Description | Acetylphosphate, also known as acetyl-p, belongs to the class of organic compounds known as acyl monophosphates. These are organic compounds containing a monophosphate linked to an acyl group. They have the general structure R-CO-P(O)(O)OH, R=H or organyl. Since acetylphosphate synthesis is known to depend on cholinesterase activity, pseudocholinesterase was assumed to participate to a small extent in acetylphosphate synthesis by cancerous serum. It is also an intermediate in pyruvate metabolism. Acetylphosphate is a drug. Acetylphosphate exists in all living organisms, ranging from bacteria to humans. Acetylphosphate can be converted into acetic acid; which is mediated by the enzyme acylphosphatase-1. It is generated from pyruvate and the formation is catalyzed by pyruvate oxidase (EC:1.2.3.3). In humans, acetylphosphate is involved in the metabolic disorder called the pyruvate dehydrogenase complex deficiency pathway. It is generated from sulfoacetaldehyde, converted to acetyl-CoA and acetate via phosphate acetyltransferase (EC:2.3.1.8) and acetate kinase (EC:2.7.2.1) respectively. Acetylphosphate or actyl phosphate is a compound involved in taurine and hypotaurine metabolism as well as pyruvate metabolism. Cancerous serum produced 37% less acetylphosphate than normal serum. |
|---|
| Structure | InChI=1S/C2H5O5P/c1-2(3)7-8(4,5)6/h1H3,(H2,4,5,6) |
|---|
| Synonyms | | Value | Source |
|---|
| Acetic acid, monoanhydride with phosphoric acid | ChEBI | | Acetyl phosphate | ChEBI | | Acetylphosphoric acid | ChEBI | | Monoacetyl phosphate | ChEBI | | Acetate, monoanhydride with phosphate | Generator | | Acetyl phosphoric acid | Generator | | Monoacetyl phosphoric acid | Generator | | Acetyl-p | HMDB |
|
|---|
| Chemical Formula | C2H5O5P |
|---|
| Average Molecular Weight | 140.0319 |
|---|
| Monoisotopic Molecular Weight | 139.987459782 |
|---|
| IUPAC Name | (acetyloxy)phosphonic acid |
|---|
| Traditional Name | acetylphosphate |
|---|
| CAS Registry Number | 590-54-5 |
|---|
| SMILES | CC(=O)OP(O)(O)=O |
|---|
| InChI Identifier | InChI=1S/C2H5O5P/c1-2(3)7-8(4,5)6/h1H3,(H2,4,5,6) |
|---|
| InChI Key | LIPOUNRJVLNBCD-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as acyl monophosphates. These are organic compounds containing a monophosphate linked to an acyl group. They have the general structure R-CO-P(O)(O)OH, R=H or organyl. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Organic phosphoric acids and derivatives |
|---|
| Sub Class | Phosphate esters |
|---|
| Direct Parent | Acyl monophosphates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Acyl monophosphate
- Acetate salt
- Carboxylic acid salt
- Monocarboxylic acid or derivatives
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organic salt
- Organooxygen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Physiological effect | Not Available |
|---|
| Disposition | |
|---|
| Process | |
|---|
| Role | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Not Available |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Retention Times Underivatized| Chromatographic Method | Retention Time | Reference |
|---|
| Measured using a Waters Acquity ultraperformance liquid chromatography (UPLC) ethylene-bridged hybrid (BEH) C18 column (100 mm × 2.1 mm; 1.7 μmparticle diameter). Predicted by Afia on May 17, 2022. Predicted by Afia on May 17, 2022. | 2.57 minutes | 32390414 | | Predicted by Siyang on May 30, 2022 | 8.6526 minutes | 33406817 | | Predicted by Siyang using ReTip algorithm on June 8, 2022 | 7.72 minutes | 32390414 | | AjsUoB = Accucore 150 Amide HILIC with 10mM Ammonium Formate, 0.1% Formic Acid | 388.8 seconds | 40023050 | | Fem_Long = Waters ACQUITY UPLC HSS T3 C18 with Water:MeOH and 0.1% Formic Acid | 464.1 seconds | 40023050 | | Fem_Lipids = Ascentis Express C18 with (60:40 water:ACN):(90:10 IPA:ACN) and 10mM NH4COOH + 0.1% Formic Acid | 344.6 seconds | 40023050 | | Life_Old = Waters ACQUITY UPLC BEH C18 with Water:(20:80 acetone:ACN) and 0.1% Formic Acid | 66.2 seconds | 40023050 | | Life_New = RP Waters ACQUITY UPLC HSS T3 C18 with Water:(30:70 MeOH:ACN) and 0.1% Formic Acid | 244.9 seconds | 40023050 | | RIKEN = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 92.7 seconds | 40023050 | | Eawag_XBridgeC18 = XBridge C18 3.5u 2.1x50 mm with Water:MeOH and 0.1% Formic Acid | 288.1 seconds | 40023050 | | BfG_NTS_RP1 =Agilent Zorbax Eclipse Plus C18 (2.1 mm x 150 mm, 3.5 um) with Water:ACN and 0.1% Formic Acid | 238.7 seconds | 40023050 | | HILIC_BDD_2 = Merck SeQuant ZIC-HILIC with ACN(0.1% formic acid):water(16 mM ammonium formate) | 762.8 seconds | 40023050 | | UniToyama_Atlantis = RP Waters Atlantis T3 (2.1 x 150 mm, 5 um) with ACN:Water and 0.1% Formic Acid | 580.2 seconds | 40023050 | | BDD_C18 = Hypersil Gold 1.9µm C18 with Water:ACN and 0.1% Formic Acid | 37.5 seconds | 40023050 | | UFZ_Phenomenex = Kinetex Core-Shell C18 2.6 um, 3.0 x 100 mm, Phenomenex with Water:MeOH and 0.1% Formic Acid | 600.6 seconds | 40023050 | | SNU_RIKEN_POS = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 217.8 seconds | 40023050 | | RPMMFDA = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 332.1 seconds | 40023050 | | MTBLS87 = Merck SeQuant ZIC-pHILIC column with ACN:Water and :ammonium carbonate | 717.9 seconds | 40023050 | | KI_GIAR_zic_HILIC_pH2_7 = Merck SeQuant ZIC-HILIC with ACN:Water and 0.1% FA | 421.2 seconds | 40023050 | | Meister zic-pHILIC pH9.3 = Merck SeQuant ZIC-pHILIC column with ACN:Water 5mM NH4Ac pH9.3 and 5mM ammonium acetate in water | 451.7 seconds | 40023050 |
Predicted Kovats Retention IndicesUnderivatizedDerivatized| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| Acetylphosphate,1TMS,isomer #1 | CC(=O)OP(=O)(O)O[Si](C)(C)C | 1159.4 | Semi standard non polar | 33892256 | | Acetylphosphate,1TMS,isomer #1 | CC(=O)OP(=O)(O)O[Si](C)(C)C | 1214.7 | Standard non polar | 33892256 | | Acetylphosphate,1TMS,isomer #1 | CC(=O)OP(=O)(O)O[Si](C)(C)C | 1627.9 | Standard polar | 33892256 | | Acetylphosphate,2TMS,isomer #1 | CC(=O)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C | 1257.4 | Semi standard non polar | 33892256 | | Acetylphosphate,2TMS,isomer #1 | CC(=O)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C | 1360.7 | Standard non polar | 33892256 | | Acetylphosphate,2TMS,isomer #1 | CC(=O)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C | 1422.7 | Standard polar | 33892256 | | Acetylphosphate,1TBDMS,isomer #1 | CC(=O)OP(=O)(O)O[Si](C)(C)C(C)(C)C | 1424.8 | Semi standard non polar | 33892256 | | Acetylphosphate,1TBDMS,isomer #1 | CC(=O)OP(=O)(O)O[Si](C)(C)C(C)(C)C | 1463.0 | Standard non polar | 33892256 | | Acetylphosphate,1TBDMS,isomer #1 | CC(=O)OP(=O)(O)O[Si](C)(C)C(C)(C)C | 1777.8 | Standard polar | 33892256 | | Acetylphosphate,2TBDMS,isomer #1 | CC(=O)OP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 1668.6 | Semi standard non polar | 33892256 | | Acetylphosphate,2TBDMS,isomer #1 | CC(=O)OP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 1795.6 | Standard non polar | 33892256 | | Acetylphosphate,2TBDMS,isomer #1 | CC(=O)OP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 1730.1 | Standard polar | 33892256 |
|
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - Acetylphosphate GC-MS (Non-derivatized) - 70eV, Positive | splash10-0002-9000000000-cfae3a8437091232f5d7 | 2017-08-28 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Acetylphosphate GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental LC-MS/MS | LC-MS/MS Spectrum - Acetylphosphate Quattro_QQQ 10V, Positive-QTOF (Annotated) | splash10-004i-9100000000-69d8c7734c01156379c8 | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Acetylphosphate Quattro_QQQ 25V, Positive-QTOF (Annotated) | splash10-004i-9000000000-2f22bf3e8b4d114a56d4 | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Acetylphosphate Quattro_QQQ 40V, Positive-QTOF (Annotated) | splash10-0ufr-9000000000-55ffd7f2d3597806d481 | 2012-07-24 | HMDB team, MONA | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Acetylphosphate 10V, Positive-QTOF | splash10-0002-9100000000-9815fa392fb818a6fab2 | 2017-07-26 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Acetylphosphate 20V, Positive-QTOF | splash10-0002-9000000000-f9f47252b65adfe6b7ab | 2017-07-26 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Acetylphosphate 40V, Positive-QTOF | splash10-000w-9000000000-aee29878b0aaf31375ca | 2017-07-26 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Acetylphosphate 10V, Negative-QTOF | splash10-004i-9200000000-30c93bbfd1e1c181a0bd | 2017-07-26 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Acetylphosphate 20V, Negative-QTOF | splash10-004i-9000000000-3f8df70a36020543d1d7 | 2017-07-26 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Acetylphosphate 40V, Negative-QTOF | splash10-004i-9000000000-5ee765f8651dddb71d5c | 2017-07-26 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Acetylphosphate 10V, Positive-QTOF | splash10-0002-9400000000-ca4664e711accab93652 | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Acetylphosphate 20V, Positive-QTOF | splash10-000w-9000000000-bc91304aa950da45006b | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Acetylphosphate 40V, Positive-QTOF | splash10-0006-9000000000-c51eac598b2179e94478 | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Acetylphosphate 10V, Negative-QTOF | splash10-004i-9000000000-a5e502a2627af2048a1f | 2021-09-23 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Acetylphosphate 20V, Negative-QTOF | splash10-004i-9000000000-a5e502a2627af2048a1f | 2021-09-23 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Acetylphosphate 40V, Negative-QTOF | splash10-004i-9000000000-a5e502a2627af2048a1f | 2021-09-23 | Wishart Lab | View Spectrum |
NMR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Experimental 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, experimental) | 2012-12-04 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Experimental 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, H2O, experimental) | 2012-12-05 | Wishart Lab | View Spectrum |
IR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M-H]-) | 2023-02-03 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M+H]+) | 2023-02-03 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M+Na]+) | 2023-02-03 | FELIX lab | View Spectrum |
|
|---|
| General References | - Libeer JC, Scharpe SL, Verkerk RM, Deprettere AJ, Schepens PJ: Simultaneous determination of p-aminobenzoic acid, acetyl-p-aminobenzoic acid and p-aminohippuric acid in serum and urine by capillary gas chromatography with use of a nitrogen-phosphorus detector. Clin Chim Acta. 1981 Sep 10;115(2):119-23. [PubMed:6974621 ]
- Dowling TC, Frye RF, Zemaitis MA: Simultaneous determination of p-aminohippuric acid, acetyl-p-aminohippuric acid and iothalamate in human plasma and urine by high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl. 1998 Sep 25;716(1-2):305-13. [PubMed:9824245 ]
- Elshenawy S, Pinney SE, Stuart T, Doulias PT, Zura G, Parry S, Elovitz MA, Bennett MJ, Bansal A, Strauss JF 3rd, Ischiropoulos H, Simmons RA: The Metabolomic Signature of the Placenta in Spontaneous Preterm Birth. Int J Mol Sci. 2020 Feb 4;21(3). pii: ijms21031043. doi: 10.3390/ijms21031043. [PubMed:32033212 ]
|
|---|