| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Expected but not Quantified |
|---|
| Creation Date | 2006-05-22 15:12:08 UTC |
|---|
| Update Date | 2023-02-21 17:16:28 UTC |
|---|
| HMDB ID | HMDB0002757 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Cysteic acid |
|---|
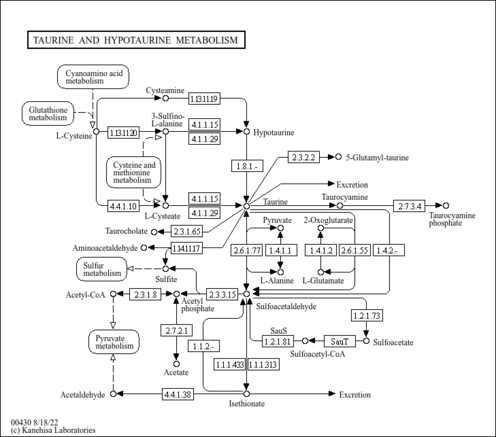
| Description | Cysteic acid, also known as cysteate or 3-sulfoalanine, belongs to the class of organic compounds known as alpha amino acids. These are amino acids in which the amino group is attached to the carbon atom immediately adjacent to the carboxylate group (alpha carbon). An amino sulfonic acid that is the sulfonic acid analogue of cysteine. Cysteic acid is a very strong basic compound (based on its pKa). Cysteic acid exists in all living species, ranging from bacteria to humans. Within humans, cysteic acid participates in a number of enzymatic reactions. In particular, cysteic acid can be converted into taurine through its interaction with the enzyme cysteine sulfinic acid decarboxylase. In addition, cysteic acid can be converted into taurine through its interaction with the enzyme glutamate decarboxylase 1. In humans, cysteic acid is involved in taurine and hypotaurine metabolism. |
|---|
| Structure | InChI=1S/C3H7NO5S/c4-2(3(5)6)1-10(7,8)9/h2H,1,4H2,(H,5,6)(H,7,8,9) |
|---|
| Synonyms | | Value | Source |
|---|
| 3-Sulfoalanine | ChEBI | | beta-Sulfoalanine | ChEBI | | Cysteine sulfonic acid | ChEBI | | 3-Sulphoalanine | Generator | | b-Sulfoalanine | Generator | | b-Sulphoalanine | Generator | | beta-Sulphoalanine | Generator | | Β-sulfoalanine | Generator | | Β-sulphoalanine | Generator | | Cysteine sulfonate | Generator | | Cysteine sulphonate | Generator | | Cysteine sulphonic acid | Generator | | Cysteate | Generator | | 2-Amino-3-sulfopropanoate | HMDB | | 2-Amino-3-sulfopropanoic acid | HMDB | | 2-Amino-3-sulfopropionate | HMDB | | 2-Amino-3-sulfopropionic acid | HMDB | | Cepteate | HMDB | | Cepteic acid | HMDB | | Cipteate | HMDB | | Cipteic acid | HMDB | | Cysteinate | HMDB | | Cysteinesulfonate | HMDB | | Cysteinesulfonic acid | HMDB | | Cysteinic acid | HMDB | | Cysterate | HMDB | | Cysteric acid | HMDB | | L-Cysteate | HMDB | | L-Cysteic acid | HMDB | | 3 Sulfoalanine | HMDB | | Acid, cysteic | HMDB | | beta Sulfoalanine | HMDB |
|
|---|
| Chemical Formula | C3H7NO5S |
|---|
| Average Molecular Weight | 169.156 |
|---|
| Monoisotopic Molecular Weight | 169.004493029 |
|---|
| IUPAC Name | 2-amino-3-sulfopropanoic acid |
|---|
| Traditional Name | cysteic acid |
|---|
| CAS Registry Number | 498-40-8 |
|---|
| SMILES | NC(CS(O)(=O)=O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C3H7NO5S/c4-2(3(5)6)1-10(7,8)9/h2H,1,4H2,(H,5,6)(H,7,8,9) |
|---|
| InChI Key | XVOYSCVBGLVSOL-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as alpha amino acids. These are amino acids in which the amino group is attached to the carbon atom immediately adjacent to the carboxylate group (alpha carbon). |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Alpha amino acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-amino acid
- Organic sulfonic acid or derivatives
- Organosulfonic acid or derivatives
- Alkanesulfonic acid
- Sulfonyl
- Organosulfonic acid
- Amino acid
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Organic nitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Primary amine
- Organosulfur compound
- Organooxygen compound
- Organonitrogen compound
- Organopnictogen compound
- Primary aliphatic amine
- Organic oxygen compound
- Carbonyl group
- Amine
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Physiological effect | Not Available |
|---|
| Disposition | |
|---|
| Process | |
|---|
| Role | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Experimental Collision Cross Sections |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Retention Times Underivatized| Chromatographic Method | Retention Time | Reference |
|---|
| Measured using a Waters Acquity ultraperformance liquid chromatography (UPLC) ethylene-bridged hybrid (BEH) C18 column (100 mm × 2.1 mm; 1.7 μmparticle diameter). Predicted by Afia on May 17, 2022. Predicted by Afia on May 17, 2022. | 0.8 minutes | 32390414 | | Predicted by Siyang on May 30, 2022 | 9.4302 minutes | 33406817 | | Predicted by Siyang using ReTip algorithm on June 8, 2022 | 9.3 minutes | 32390414 | | AjsUoB = Accucore 150 Amide HILIC with 10mM Ammonium Formate, 0.1% Formic Acid | 417.9 seconds | 40023050 | | Fem_Long = Waters ACQUITY UPLC HSS T3 C18 with Water:MeOH and 0.1% Formic Acid | 548.0 seconds | 40023050 | | Fem_Lipids = Ascentis Express C18 with (60:40 water:ACN):(90:10 IPA:ACN) and 10mM NH4COOH + 0.1% Formic Acid | 351.1 seconds | 40023050 | | Life_Old = Waters ACQUITY UPLC BEH C18 with Water:(20:80 acetone:ACN) and 0.1% Formic Acid | 34.2 seconds | 40023050 | | Life_New = RP Waters ACQUITY UPLC HSS T3 C18 with Water:(30:70 MeOH:ACN) and 0.1% Formic Acid | 222.9 seconds | 40023050 | | RIKEN = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 83.7 seconds | 40023050 | | Eawag_XBridgeC18 = XBridge C18 3.5u 2.1x50 mm with Water:MeOH and 0.1% Formic Acid | 292.8 seconds | 40023050 | | BfG_NTS_RP1 =Agilent Zorbax Eclipse Plus C18 (2.1 mm x 150 mm, 3.5 um) with Water:ACN and 0.1% Formic Acid | 218.6 seconds | 40023050 | | HILIC_BDD_2 = Merck SeQuant ZIC-HILIC with ACN(0.1% formic acid):water(16 mM ammonium formate) | 972.1 seconds | 40023050 | | UniToyama_Atlantis = RP Waters Atlantis T3 (2.1 x 150 mm, 5 um) with ACN:Water and 0.1% Formic Acid | 598.3 seconds | 40023050 | | BDD_C18 = Hypersil Gold 1.9µm C18 with Water:ACN and 0.1% Formic Acid | 39.2 seconds | 40023050 | | UFZ_Phenomenex = Kinetex Core-Shell C18 2.6 um, 3.0 x 100 mm, Phenomenex with Water:MeOH and 0.1% Formic Acid | 699.7 seconds | 40023050 | | SNU_RIKEN_POS = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 222.5 seconds | 40023050 | | RPMMFDA = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 368.7 seconds | 40023050 | | MTBLS87 = Merck SeQuant ZIC-pHILIC column with ACN:Water and :ammonium carbonate | 716.3 seconds | 40023050 | | KI_GIAR_zic_HILIC_pH2_7 = Merck SeQuant ZIC-HILIC with ACN:Water and 0.1% FA | 447.5 seconds | 40023050 | | Meister zic-pHILIC pH9.3 = Merck SeQuant ZIC-pHILIC column with ACN:Water 5mM NH4Ac pH9.3 and 5mM ammonium acetate in water | 476.6 seconds | 40023050 |
Predicted Kovats Retention IndicesUnderivatizedDerivatized| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| Cysteic acid,1TMS,isomer #1 | C[Si](C)(C)OC(=O)C(N)CS(=O)(=O)O | 1672.9 | Semi standard non polar | 33892256 | | Cysteic acid,1TMS,isomer #2 | C[Si](C)(C)OS(=O)(=O)CC(N)C(=O)O | 1717.7 | Semi standard non polar | 33892256 | | Cysteic acid,1TMS,isomer #3 | C[Si](C)(C)NC(CS(=O)(=O)O)C(=O)O | 1665.4 | Semi standard non polar | 33892256 | | Cysteic acid,2TMS,isomer #1 | C[Si](C)(C)OC(=O)C(N)CS(=O)(=O)O[Si](C)(C)C | 1724.6 | Semi standard non polar | 33892256 | | Cysteic acid,2TMS,isomer #1 | C[Si](C)(C)OC(=O)C(N)CS(=O)(=O)O[Si](C)(C)C | 1696.1 | Standard non polar | 33892256 | | Cysteic acid,2TMS,isomer #1 | C[Si](C)(C)OC(=O)C(N)CS(=O)(=O)O[Si](C)(C)C | 2822.4 | Standard polar | 33892256 | | Cysteic acid,2TMS,isomer #2 | C[Si](C)(C)NC(CS(=O)(=O)O)C(=O)O[Si](C)(C)C | 1747.0 | Semi standard non polar | 33892256 | | Cysteic acid,2TMS,isomer #2 | C[Si](C)(C)NC(CS(=O)(=O)O)C(=O)O[Si](C)(C)C | 1745.4 | Standard non polar | 33892256 | | Cysteic acid,2TMS,isomer #2 | C[Si](C)(C)NC(CS(=O)(=O)O)C(=O)O[Si](C)(C)C | 2721.2 | Standard polar | 33892256 | | Cysteic acid,2TMS,isomer #3 | C[Si](C)(C)NC(CS(=O)(=O)O[Si](C)(C)C)C(=O)O | 1798.4 | Semi standard non polar | 33892256 | | Cysteic acid,2TMS,isomer #3 | C[Si](C)(C)NC(CS(=O)(=O)O[Si](C)(C)C)C(=O)O | 1741.5 | Standard non polar | 33892256 | | Cysteic acid,2TMS,isomer #3 | C[Si](C)(C)NC(CS(=O)(=O)O[Si](C)(C)C)C(=O)O | 2558.1 | Standard polar | 33892256 | | Cysteic acid,2TMS,isomer #4 | C[Si](C)(C)N(C(CS(=O)(=O)O)C(=O)O)[Si](C)(C)C | 1847.8 | Semi standard non polar | 33892256 | | Cysteic acid,2TMS,isomer #4 | C[Si](C)(C)N(C(CS(=O)(=O)O)C(=O)O)[Si](C)(C)C | 1892.4 | Standard non polar | 33892256 | | Cysteic acid,2TMS,isomer #4 | C[Si](C)(C)N(C(CS(=O)(=O)O)C(=O)O)[Si](C)(C)C | 2799.7 | Standard polar | 33892256 | | Cysteic acid,3TMS,isomer #1 | C[Si](C)(C)NC(CS(=O)(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C | 1814.8 | Semi standard non polar | 33892256 | | Cysteic acid,3TMS,isomer #1 | C[Si](C)(C)NC(CS(=O)(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C | 1908.8 | Standard non polar | 33892256 | | Cysteic acid,3TMS,isomer #1 | C[Si](C)(C)NC(CS(=O)(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C | 2241.3 | Standard polar | 33892256 | | Cysteic acid,3TMS,isomer #2 | C[Si](C)(C)OC(=O)C(CS(=O)(=O)O)N([Si](C)(C)C)[Si](C)(C)C | 1891.7 | Semi standard non polar | 33892256 | | Cysteic acid,3TMS,isomer #2 | C[Si](C)(C)OC(=O)C(CS(=O)(=O)O)N([Si](C)(C)C)[Si](C)(C)C | 2023.8 | Standard non polar | 33892256 | | Cysteic acid,3TMS,isomer #2 | C[Si](C)(C)OC(=O)C(CS(=O)(=O)O)N([Si](C)(C)C)[Si](C)(C)C | 2399.4 | Standard polar | 33892256 | | Cysteic acid,3TMS,isomer #3 | C[Si](C)(C)OS(=O)(=O)CC(C(=O)O)N([Si](C)(C)C)[Si](C)(C)C | 1955.1 | Semi standard non polar | 33892256 | | Cysteic acid,3TMS,isomer #3 | C[Si](C)(C)OS(=O)(=O)CC(C(=O)O)N([Si](C)(C)C)[Si](C)(C)C | 2011.8 | Standard non polar | 33892256 | | Cysteic acid,3TMS,isomer #3 | C[Si](C)(C)OS(=O)(=O)CC(C(=O)O)N([Si](C)(C)C)[Si](C)(C)C | 2342.9 | Standard polar | 33892256 | | Cysteic acid,4TMS,isomer #1 | C[Si](C)(C)OC(=O)C(CS(=O)(=O)O[Si](C)(C)C)N([Si](C)(C)C)[Si](C)(C)C | 1980.5 | Semi standard non polar | 33892256 | | Cysteic acid,4TMS,isomer #1 | C[Si](C)(C)OC(=O)C(CS(=O)(=O)O[Si](C)(C)C)N([Si](C)(C)C)[Si](C)(C)C | 2159.8 | Standard non polar | 33892256 | | Cysteic acid,4TMS,isomer #1 | C[Si](C)(C)OC(=O)C(CS(=O)(=O)O[Si](C)(C)C)N([Si](C)(C)C)[Si](C)(C)C | 2159.6 | Standard polar | 33892256 | | Cysteic acid,1TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)C(N)CS(=O)(=O)O | 1937.9 | Semi standard non polar | 33892256 | | Cysteic acid,1TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)OS(=O)(=O)CC(N)C(=O)O | 1952.3 | Semi standard non polar | 33892256 | | Cysteic acid,1TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)NC(CS(=O)(=O)O)C(=O)O | 1936.1 | Semi standard non polar | 33892256 | | Cysteic acid,2TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)C(N)CS(=O)(=O)O[Si](C)(C)C(C)(C)C | 2201.6 | Semi standard non polar | 33892256 | | Cysteic acid,2TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)C(N)CS(=O)(=O)O[Si](C)(C)C(C)(C)C | 2272.5 | Standard non polar | 33892256 | | Cysteic acid,2TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)C(N)CS(=O)(=O)O[Si](C)(C)C(C)(C)C | 2891.6 | Standard polar | 33892256 | | Cysteic acid,2TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)NC(CS(=O)(=O)O)C(=O)O[Si](C)(C)C(C)(C)C | 2227.9 | Semi standard non polar | 33892256 | | Cysteic acid,2TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)NC(CS(=O)(=O)O)C(=O)O[Si](C)(C)C(C)(C)C | 2282.5 | Standard non polar | 33892256 | | Cysteic acid,2TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)NC(CS(=O)(=O)O)C(=O)O[Si](C)(C)C(C)(C)C | 2764.4 | Standard polar | 33892256 | | Cysteic acid,2TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)NC(CS(=O)(=O)O[Si](C)(C)C(C)(C)C)C(=O)O | 2284.5 | Semi standard non polar | 33892256 | | Cysteic acid,2TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)NC(CS(=O)(=O)O[Si](C)(C)C(C)(C)C)C(=O)O | 2313.3 | Standard non polar | 33892256 | | Cysteic acid,2TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)NC(CS(=O)(=O)O[Si](C)(C)C(C)(C)C)C(=O)O | 2628.3 | Standard polar | 33892256 | | Cysteic acid,2TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)N(C(CS(=O)(=O)O)C(=O)O)[Si](C)(C)C(C)(C)C | 2299.2 | Semi standard non polar | 33892256 | | Cysteic acid,2TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)N(C(CS(=O)(=O)O)C(=O)O)[Si](C)(C)C(C)(C)C | 2387.6 | Standard non polar | 33892256 | | Cysteic acid,2TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)N(C(CS(=O)(=O)O)C(=O)O)[Si](C)(C)C(C)(C)C | 2767.5 | Standard polar | 33892256 | | Cysteic acid,3TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)NC(CS(=O)(=O)O[Si](C)(C)C(C)(C)C)C(=O)O[Si](C)(C)C(C)(C)C | 2445.0 | Semi standard non polar | 33892256 | | Cysteic acid,3TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)NC(CS(=O)(=O)O[Si](C)(C)C(C)(C)C)C(=O)O[Si](C)(C)C(C)(C)C | 2741.6 | Standard non polar | 33892256 | | Cysteic acid,3TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)NC(CS(=O)(=O)O[Si](C)(C)C(C)(C)C)C(=O)O[Si](C)(C)C(C)(C)C | 2515.6 | Standard polar | 33892256 | | Cysteic acid,3TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)OC(=O)C(CS(=O)(=O)O)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2534.3 | Semi standard non polar | 33892256 | | Cysteic acid,3TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)OC(=O)C(CS(=O)(=O)O)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2757.2 | Standard non polar | 33892256 | | Cysteic acid,3TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)OC(=O)C(CS(=O)(=O)O)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2592.4 | Standard polar | 33892256 | | Cysteic acid,3TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OS(=O)(=O)CC(C(=O)O)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2562.1 | Semi standard non polar | 33892256 | | Cysteic acid,3TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OS(=O)(=O)CC(C(=O)O)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2835.8 | Standard non polar | 33892256 | | Cysteic acid,3TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OS(=O)(=O)CC(C(=O)O)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2548.9 | Standard polar | 33892256 | | Cysteic acid,4TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)C(CS(=O)(=O)O[Si](C)(C)C(C)(C)C)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2780.5 | Semi standard non polar | 33892256 | | Cysteic acid,4TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)C(CS(=O)(=O)O[Si](C)(C)C(C)(C)C)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 3235.1 | Standard non polar | 33892256 | | Cysteic acid,4TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)C(CS(=O)(=O)O[Si](C)(C)C(C)(C)C)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2487.6 | Standard polar | 33892256 |
|
|---|