| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Expected but not Quantified |
|---|
| Creation Date | 2008-08-12 14:21:20 UTC |
|---|
| Update Date | 2022-03-07 02:49:33 UTC |
|---|
| HMDB ID | HMDB0006816 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 3-Hexaprenyl-4-hydroxybenzoic acid |
|---|
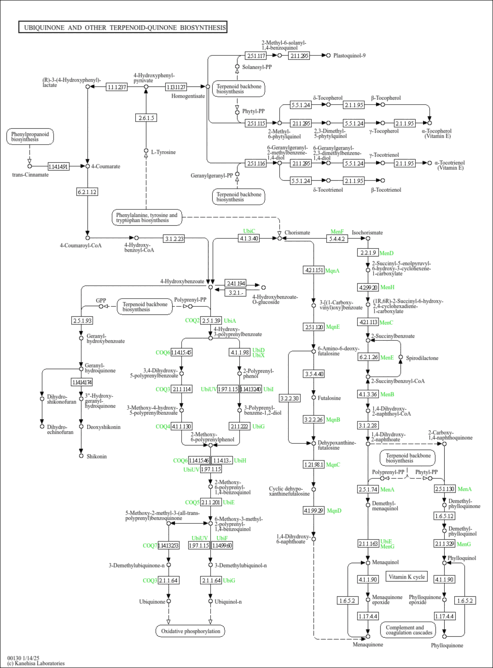
| Description | 3-Hexaprenyl-4-hydroxybenzoic acid belongs to the class of organic compounds known as polyprenylphenols. Polyprenylphenols are compounds containing a polyisoprene chain attached to a phenol group. 3-Hexaprenyl-4-hydroxybenzoic acid is a very hydrophobic molecule, practically insoluble (in water), and relatively neutral. |
|---|
| Structure | CC(C)=CCC\C(C)=C\CC\C(C)=C\CC\C(C)=C\CC\C(C)=C\CC\C(C)=C\CC1=CC(=CC=C1O)C(O)=O InChI=1S/C37H54O3/c1-28(2)13-8-14-29(3)15-9-16-30(4)17-10-18-31(5)19-11-20-32(6)21-12-22-33(7)23-24-34-27-35(37(39)40)25-26-36(34)38/h13,15,17,19,21,23,25-27,38H,8-12,14,16,18,20,22,24H2,1-7H3,(H,39,40)/b29-15+,30-17+,31-19+,32-21+,33-23+ |
|---|
| Synonyms | | Value | Source |
|---|
| (all-e)-3-(3,7,11,15,19,23-Hexamethyl-2,6,10,14,18,22-tetracosahexaenyl)-4-hydroxybenzoic acid | ChEBI | | all-trans-3-(3,7,11,15,19,23-Hexamethyl-2,6,10,14,18,22-tetracosahexaenyl)-4-hydroxybenzoic acid | ChEBI | | (all-e)-3-(3,7,11,15,19,23-Hexamethyl-2,6,10,14,18,22-tetracosahexaenyl)-4-hydroxybenzoate | Generator | | all-trans-3-(3,7,11,15,19,23-Hexamethyl-2,6,10,14,18,22-tetracosahexaenyl)-4-hydroxybenzoate | Generator | | 3-Hexaprenyl-4-hydroxybenzoate | Generator | | 3-(3,7,11,15,19,23-Hexamethyl-2,6,10,14,18,22-tetracosahexaenyl)-4-hydroxy-benzoate | HMDB | | 3-(3,7,11,15,19,23-Hexamethyl-2,6,10,14,18,22-tetracosahexaenyl)-4-hydroxy-benzoic acid | HMDB |
|
|---|
| Chemical Formula | C37H54O3 |
|---|
| Average Molecular Weight | 546.8229 |
|---|
| Monoisotopic Molecular Weight | 546.407295594 |
|---|
| IUPAC Name | 3-[(2E,6E,10E,14E,18E)-3,7,11,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaen-1-yl]-4-hydroxybenzoic acid |
|---|
| Traditional Name | 3-hexaprenyl-4-hydroxybenzoate |
|---|
| CAS Registry Number | 65848-03-5 |
|---|
| SMILES | CC(C)=CCC\C(C)=C\CC\C(C)=C\CC\C(C)=C\CC\C(C)=C\CC\C(C)=C\CC1=CC(=CC=C1O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C37H54O3/c1-28(2)13-8-14-29(3)15-9-16-30(4)17-10-18-31(5)19-11-20-32(6)21-12-22-33(7)23-24-34-27-35(37(39)40)25-26-36(34)38/h13,15,17,19,21,23,25-27,38H,8-12,14,16,18,20,22,24H2,1-7H3,(H,39,40)/b29-15+,30-17+,31-19+,32-21+,33-23+ |
|---|
| InChI Key | LKMQQQABIGIHGL-LAAQXVIISA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as polyprenylphenols. Polyprenylphenols are compounds containing a polyisoprene chain attached to a phenol group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Polyprenylphenols |
|---|
| Direct Parent | Polyprenylphenols |
|---|
| Alternative Parents | |
|---|
| Substituents | - Polyprenylphenol
- Sesterterpenoid
- Hydroxybenzoic acid
- Benzoic acid or derivatives
- Benzoic acid
- Benzoyl
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Monocyclic benzene moiety
- Benzenoid
- Carboxylic acid
- Carboxylic acid derivative
- Monocarboxylic acid or derivatives
- Hydrocarbon derivative
- Organic oxide
- Organic oxygen compound
- Organooxygen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Physiological effect | Not Available |
|---|
| Disposition | |
|---|
| Process | |
|---|
| Role | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Not Available |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Kovats Retention IndicesUnderivatizedDerivatized| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| 3-Hexaprenyl-4-hydroxybenzoic acid,1TMS,isomer #1 | CC(C)=CCC/C(C)=C/CC/C(C)=C/CC/C(C)=C/CC/C(C)=C/CC/C(C)=C/CC1=CC(C(=O)O)=CC=C1O[Si](C)(C)C | 4206.3 | Semi standard non polar | 33892256 | | 3-Hexaprenyl-4-hydroxybenzoic acid,1TMS,isomer #2 | CC(C)=CCC/C(C)=C/CC/C(C)=C/CC/C(C)=C/CC/C(C)=C/CC/C(C)=C/CC1=CC(C(=O)O[Si](C)(C)C)=CC=C1O | 4094.6 | Semi standard non polar | 33892256 | | 3-Hexaprenyl-4-hydroxybenzoic acid,2TMS,isomer #1 | CC(C)=CCC/C(C)=C/CC/C(C)=C/CC/C(C)=C/CC/C(C)=C/CC/C(C)=C/CC1=CC(C(=O)O[Si](C)(C)C)=CC=C1O[Si](C)(C)C | 4038.1 | Semi standard non polar | 33892256 | | 3-Hexaprenyl-4-hydroxybenzoic acid,1TBDMS,isomer #1 | CC(C)=CCC/C(C)=C/CC/C(C)=C/CC/C(C)=C/CC/C(C)=C/CC/C(C)=C/CC1=CC(C(=O)O)=CC=C1O[Si](C)(C)C(C)(C)C | 4438.6 | Semi standard non polar | 33892256 | | 3-Hexaprenyl-4-hydroxybenzoic acid,1TBDMS,isomer #2 | CC(C)=CCC/C(C)=C/CC/C(C)=C/CC/C(C)=C/CC/C(C)=C/CC/C(C)=C/CC1=CC(C(=O)O[Si](C)(C)C(C)(C)C)=CC=C1O | 4332.1 | Semi standard non polar | 33892256 | | 3-Hexaprenyl-4-hydroxybenzoic acid,2TBDMS,isomer #1 | CC(C)=CCC/C(C)=C/CC/C(C)=C/CC/C(C)=C/CC/C(C)=C/CC/C(C)=C/CC1=CC(C(=O)O[Si](C)(C)C(C)(C)C)=CC=C1O[Si](C)(C)C(C)(C)C | 4435.5 | Semi standard non polar | 33892256 |
|
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - 3-Hexaprenyl-4-hydroxybenzoic acid GC-MS (Non-derivatized) - 70eV, Positive | splash10-05ai-3496450000-5b468805ae29a5f59a42 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 3-Hexaprenyl-4-hydroxybenzoic acid GC-MS (2 TMS) - 70eV, Positive | splash10-004i-3222219000-0638b89fa0bde34d7abb | 2017-10-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 3-Hexaprenyl-4-hydroxybenzoic acid GC-MS ("3-Hexaprenyl-4-hydroxybenzoic acid,1TMS,#1" TMS) - 70eV, Positive | Not Available | 2021-10-14 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 3-Hexaprenyl-4-hydroxybenzoic acid GC-MS (TMS_1_1) - 70eV, Positive | Not Available | 2021-10-14 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 3-Hexaprenyl-4-hydroxybenzoic acid GC-MS (TMS_1_2) - 70eV, Positive | Not Available | 2021-10-14 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 3-Hexaprenyl-4-hydroxybenzoic acid GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | 2021-10-14 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 3-Hexaprenyl-4-hydroxybenzoic acid GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | 2021-10-14 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 3-Hexaprenyl-4-hydroxybenzoic acid GC-MS (TBDMS_2_1) - 70eV, Positive | Not Available | 2021-10-14 | Wishart Lab | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3-Hexaprenyl-4-hydroxybenzoic acid 10V, Positive-QTOF | splash10-0f92-0111090000-0efba026d858a4ae0c57 | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3-Hexaprenyl-4-hydroxybenzoic acid 20V, Positive-QTOF | splash10-0ufs-0749360000-6112d4d11f00cf6a1fbd | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3-Hexaprenyl-4-hydroxybenzoic acid 40V, Positive-QTOF | splash10-0002-2579200000-1a695b7874053d38599a | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3-Hexaprenyl-4-hydroxybenzoic acid 10V, Negative-QTOF | splash10-0002-0000090000-451426c2227e9a66c0ae | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3-Hexaprenyl-4-hydroxybenzoic acid 20V, Negative-QTOF | splash10-0udj-0000190000-4173c10b0b5d3133deab | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3-Hexaprenyl-4-hydroxybenzoic acid 40V, Negative-QTOF | splash10-002r-2112920000-b9b9e484d8dc60509991 | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3-Hexaprenyl-4-hydroxybenzoic acid 10V, Positive-QTOF | splash10-002b-1102590000-0baf32808b3ddee5f52b | 2021-09-23 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3-Hexaprenyl-4-hydroxybenzoic acid 20V, Positive-QTOF | splash10-0uy0-1629600000-89c41d697dba8ac52603 | 2021-09-23 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3-Hexaprenyl-4-hydroxybenzoic acid 40V, Positive-QTOF | splash10-0pbi-0859510000-223193ff35c3e152d619 | 2021-09-23 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3-Hexaprenyl-4-hydroxybenzoic acid 10V, Negative-QTOF | splash10-0002-0000090000-d1b5f9d61cd518fa226b | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3-Hexaprenyl-4-hydroxybenzoic acid 20V, Negative-QTOF | splash10-052b-0900010000-73c408ecbea0457640ca | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3-Hexaprenyl-4-hydroxybenzoic acid 40V, Negative-QTOF | splash10-001i-1403900000-b753301bd29d4e9b1925 | 2021-09-25 | Wishart Lab | View Spectrum |
|
|---|