| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Expected but not Quantified |
|---|
| Creation Date | 2008-08-13 10:45:47 UTC |
|---|
| Update Date | 2022-03-07 02:49:33 UTC |
|---|
| HMDB ID | HMDB0006838 |
|---|
| Secondary Accession Numbers | - HMDB0006923
- HMDB06838
- HMDB06923
|
|---|
| Metabolite Identification |
|---|
| Common Name | 3-Keto-4-methylzymosterol |
|---|
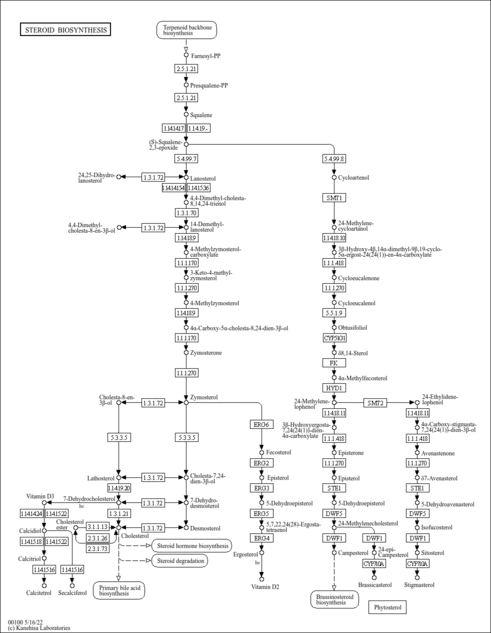
| Description | 3-Keto-4-methylzymosterol is an intermediate in the biosynthesis of steroids (KEGG:C15816). It is the 8th to last step in the synthesis of vitamin D2 and is converted from 4-methtylzymosterol-carboxylate via the enzyme sterol-4alpha-carboxylate 3-dehydrogenase (decarboxylating) (EC:1.1.1.170). It is then converted to 4-methylzymosterol via the enzyme 3-keto steroid reductase (EC:1.1.1.270). |
|---|
| Structure | C[C@H](CCC=C(C)C)C1CCC2C3=C(CC[C@]12C)[C@@]1(C)CCC(=O)C(C)C1CC3 InChI=1S/C28H44O/c1-18(2)8-7-9-19(3)22-12-13-24-21-10-11-23-20(4)26(29)15-17-28(23,6)25(21)14-16-27(22,24)5/h8,19-20,22-24H,7,9-17H2,1-6H3/t19-,20?,22?,23?,24?,27-,28+/m1/s1 |
|---|
| Synonyms | | Value | Source |
|---|
| (5a)-4-Methylcholesta-8,24-dien-3-one | HMDB | | (5alpha)-4-Methylcholesta-8,24-dien-3-one | HMDB | | 3-Dehydro-4-methylzymosterol | HMDB | | 4-Methyl-5a-cholesta-8,24-dien-3-one | HMDB | | 4-Methyl-5alpha-cholesta-8,24-dien-3-one | HMDB |
|
|---|
| Chemical Formula | C28H44O |
|---|
| Average Molecular Weight | 396.6484 |
|---|
| Monoisotopic Molecular Weight | 396.33921603 |
|---|
| IUPAC Name | (2S,15R)-2,6,15-trimethyl-14-[(2R)-6-methylhept-5-en-2-yl]tetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-1(10)-en-5-one |
|---|
| Traditional Name | (2S,15R)-2,6,15-trimethyl-14-[(2R)-6-methylhept-5-en-2-yl]tetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-1(10)-en-5-one |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | C[C@H](CCC=C(C)C)C1CCC2C3=C(CC[C@]12C)[C@@]1(C)CCC(=O)C(C)C1CC3 |
|---|
| InChI Identifier | InChI=1S/C28H44O/c1-18(2)8-7-9-19(3)22-12-13-24-21-10-11-23-20(4)26(29)15-17-28(23,6)25(21)14-16-27(22,24)5/h8,19-20,22-24H,7,9-17H2,1-6H3/t19-,20?,22?,23?,24?,27-,28+/m1/s1 |
|---|
| InChI Key | DBPZYKHQDWKORQ-JQDPPCHWSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as cholesterols and derivatives. Cholesterols and derivatives are compounds containing a 3-hydroxylated cholestane core. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Cholestane steroids |
|---|
| Direct Parent | Cholesterols and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Cholesterol-skeleton
- Oxosteroid
- 3-oxosteroid
- Cyclic ketone
- Ketone
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Physiological effect | |
|---|
| Disposition | |
|---|
| Process | |
|---|
| Role | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Not Available |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Kovats Retention IndicesUnderivatizedDerivatized| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| 3-Keto-4-methylzymosterol,1TMS,isomer #1 | CC(C)=CCC[C@@H](C)C1CCC2C3=C(CC[C@@]21C)[C@@]1(C)CCC(O[Si](C)(C)C)=C(C)C1CC3 | 3298.8 | Semi standard non polar | 33892256 | | 3-Keto-4-methylzymosterol,1TMS,isomer #1 | CC(C)=CCC[C@@H](C)C1CCC2C3=C(CC[C@@]21C)[C@@]1(C)CCC(O[Si](C)(C)C)=C(C)C1CC3 | 3178.0 | Standard non polar | 33892256 | | 3-Keto-4-methylzymosterol,1TMS,isomer #1 | CC(C)=CCC[C@@H](C)C1CCC2C3=C(CC[C@@]21C)[C@@]1(C)CCC(O[Si](C)(C)C)=C(C)C1CC3 | 3427.5 | Standard polar | 33892256 | | 3-Keto-4-methylzymosterol,1TMS,isomer #2 | CC(C)=CCC[C@@H](C)C1CCC2C3=C(CC[C@@]21C)[C@@]1(C)CC=C(O[Si](C)(C)C)C(C)C1CC3 | 3265.7 | Semi standard non polar | 33892256 | | 3-Keto-4-methylzymosterol,1TMS,isomer #2 | CC(C)=CCC[C@@H](C)C1CCC2C3=C(CC[C@@]21C)[C@@]1(C)CC=C(O[Si](C)(C)C)C(C)C1CC3 | 3131.0 | Standard non polar | 33892256 | | 3-Keto-4-methylzymosterol,1TMS,isomer #2 | CC(C)=CCC[C@@H](C)C1CCC2C3=C(CC[C@@]21C)[C@@]1(C)CC=C(O[Si](C)(C)C)C(C)C1CC3 | 3416.1 | Standard polar | 33892256 | | 3-Keto-4-methylzymosterol,1TBDMS,isomer #1 | CC(C)=CCC[C@@H](C)C1CCC2C3=C(CC[C@@]21C)[C@@]1(C)CCC(O[Si](C)(C)C(C)(C)C)=C(C)C1CC3 | 3505.4 | Semi standard non polar | 33892256 | | 3-Keto-4-methylzymosterol,1TBDMS,isomer #1 | CC(C)=CCC[C@@H](C)C1CCC2C3=C(CC[C@@]21C)[C@@]1(C)CCC(O[Si](C)(C)C(C)(C)C)=C(C)C1CC3 | 3423.3 | Standard non polar | 33892256 | | 3-Keto-4-methylzymosterol,1TBDMS,isomer #1 | CC(C)=CCC[C@@H](C)C1CCC2C3=C(CC[C@@]21C)[C@@]1(C)CCC(O[Si](C)(C)C(C)(C)C)=C(C)C1CC3 | 3552.0 | Standard polar | 33892256 | | 3-Keto-4-methylzymosterol,1TBDMS,isomer #2 | CC(C)=CCC[C@@H](C)C1CCC2C3=C(CC[C@@]21C)[C@@]1(C)CC=C(O[Si](C)(C)C(C)(C)C)C(C)C1CC3 | 3500.5 | Semi standard non polar | 33892256 | | 3-Keto-4-methylzymosterol,1TBDMS,isomer #2 | CC(C)=CCC[C@@H](C)C1CCC2C3=C(CC[C@@]21C)[C@@]1(C)CC=C(O[Si](C)(C)C(C)(C)C)C(C)C1CC3 | 3302.0 | Standard non polar | 33892256 | | 3-Keto-4-methylzymosterol,1TBDMS,isomer #2 | CC(C)=CCC[C@@H](C)C1CCC2C3=C(CC[C@@]21C)[C@@]1(C)CC=C(O[Si](C)(C)C(C)(C)C)C(C)C1CC3 | 3539.1 | Standard polar | 33892256 |
|
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - 3-Keto-4-methylzymosterol GC-MS (Non-derivatized) - 70eV, Positive | splash10-02au-2019000000-5173500b11e18ce35b3b | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 3-Keto-4-methylzymosterol GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 3-Keto-4-methylzymosterol GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3-Keto-4-methylzymosterol 10V, Positive-QTOF | splash10-0002-0009000000-00d160bd3605d4c8b846 | 2016-09-14 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3-Keto-4-methylzymosterol 20V, Positive-QTOF | splash10-00pv-1119000000-e5a9171fb6049ee63431 | 2016-09-14 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3-Keto-4-methylzymosterol 40V, Positive-QTOF | splash10-015i-2119000000-6b021eb86e51393d9798 | 2016-09-14 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3-Keto-4-methylzymosterol 10V, Negative-QTOF | splash10-0002-0009000000-e91a27c7e4df70239c24 | 2016-09-14 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3-Keto-4-methylzymosterol 20V, Negative-QTOF | splash10-0002-0009000000-766b7ce92b3bc6de20e7 | 2016-09-14 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3-Keto-4-methylzymosterol 40V, Negative-QTOF | splash10-01t9-3009000000-b4ba7e91fae9c3e7a4ba | 2016-09-14 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3-Keto-4-methylzymosterol 10V, Positive-QTOF | splash10-002b-0019000000-f87374bf70bdfa4a699f | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3-Keto-4-methylzymosterol 20V, Positive-QTOF | splash10-0ap0-3259000000-e97d8860c45e22b2a89d | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3-Keto-4-methylzymosterol 40V, Positive-QTOF | splash10-0a4i-6951000000-99c4649de525703330a7 | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3-Keto-4-methylzymosterol 10V, Negative-QTOF | splash10-0002-0009000000-2f6aa9af0bd8878fb6c6 | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3-Keto-4-methylzymosterol 20V, Negative-QTOF | splash10-0002-0009000000-2f6aa9af0bd8878fb6c6 | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3-Keto-4-methylzymosterol 40V, Negative-QTOF | splash10-0007-0009000000-3e9131f464f27a3024a5 | 2021-09-24 | Wishart Lab | View Spectrum |
NMR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted 1D NMR | 13C NMR Spectrum (1D, 100 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 100 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 200 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 200 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 300 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 300 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 400 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 400 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 500 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 500 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 600 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 700 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 700 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 800 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 800 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 900 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 900 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum |
|
|---|