| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Expected but not Quantified |
|---|
| Creation Date | 2009-06-18 12:29:07 UTC |
|---|
| Update Date | 2022-03-07 02:51:25 UTC |
|---|
| HMDB ID | HMDB0012452 |
|---|
| Secondary Accession Numbers | - HMDB0061095
- HMDB12452
- HMDB61095
|
|---|
| Metabolite Identification |
|---|
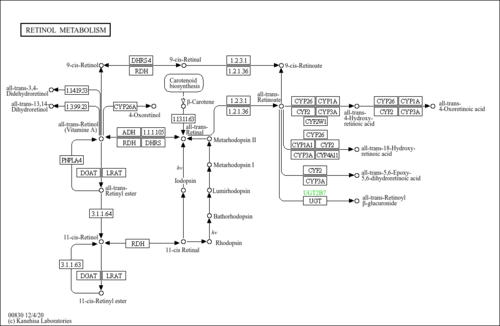
| Common Name | all-trans-18-Hydroxyretinoic acid |
|---|
| Description | all-trans-18-Hydroxyretinoic acid, also known as 18-hydroxyretinoic acid, is classified as a member of the retinoids. Retinoids are oxygenated derivatives of 3,7-dimethyl-1-(2,6,6-trimethylcyclohex-1-enyl)nona-1,3,5,7-tetraene and derivatives thereof. all-trans-18-Hydroxyretinoic acid is considered to be a practically insoluble (in water) and a weakly acidic compound. all-trans-18-Hydroxyretinoic acid is an isoprenoid lipid molecule. Within a cell, all-trans-18-hydroxyretinoic acid is primarily located in the extracellular space and near the membrane. 18-Hydroxyretinoic acid is a metabolite of tretinoin. Tretinoin is the acid form of vitamin A and is also known as all-trans retinoic acid or ATRA. It is a drug commonly used to treat acne vulgaris and keratosis pilaris. It is available as a cream or gel (brand names Aberela, Airol, Renova, Atralin, Retin-A, Avita, Retacnyl, Refissa, or Stieva-A). It is also used to treat acute promyelocytic leukemia (APL) and is sold for this indication by Roche under the brand name Vesanoid. It is also available as a generic. (Wikipedia) |
|---|
| Structure | C\C(\C=C\C1=C(CO)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O InChI=1S/C20H28O3/c1-15(7-5-8-16(2)13-19(22)23)10-11-18-17(14-21)9-6-12-20(18,3)4/h5,7-8,10-11,13,21H,6,9,12,14H2,1-4H3,(H,22,23)/b8-5+,11-10+,15-7+,16-13+ |
|---|
| Synonyms | | Value | Source |
|---|
| 18-Hydroxy-all-trans-retinoic acid | ChEBI | | 18-Hydroxyretinoic acid | ChEBI | | all-trans-9-(2-(Hydroxymethyl)-6,6-dimethyl-1-cyclohexen-1-yl)-3,7-dimethyl-2,4,6,8-nonatetraenoic acid | ChEBI | | 18-Hydroxy-all-trans-retinoate | Generator | | 18-Hydroxyretinoate | Generator | | all-trans-9-(2-(Hydroxymethyl)-6,6-dimethyl-1-cyclohexen-1-yl)-3,7-dimethyl-2,4,6,8-nonatetraenoate | Generator | | all-trans-18-Hydroxyretinoate | Generator | | rac-18-Hydroxy-all-trans-retinoate | HMDB | | rac-18-Hydroxy-all-trans-retinoic acid | HMDB | | all-trans-18-Hydroxyretinoic acid | HMDB |
|
|---|
| Chemical Formula | C20H28O3 |
|---|
| Average Molecular Weight | 316.4345 |
|---|
| Monoisotopic Molecular Weight | 316.203844762 |
|---|
| IUPAC Name | (2E,4E,6E,8E)-9-[2-(hydroxymethyl)-6,6-dimethylcyclohex-1-en-1-yl]-3,7-dimethylnona-2,4,6,8-tetraenoic acid |
|---|
| Traditional Name | (2E,4E,6E,8E)-9-[2-(hydroxymethyl)-6,6-dimethylcyclohex-1-en-1-yl]-3,7-dimethylnona-2,4,6,8-tetraenoic acid |
|---|
| CAS Registry Number | 63531-93-1 |
|---|
| SMILES | C\C(\C=C\C1=C(CO)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |
|---|
| InChI Identifier | InChI=1S/C20H28O3/c1-15(7-5-8-16(2)13-19(22)23)10-11-18-17(14-21)9-6-12-20(18,3)4/h5,7-8,10-11,13,21H,6,9,12,14H2,1-4H3,(H,22,23)/b8-5+,11-10+,15-7+,16-13+ |
|---|
| InChI Key | XSJOIRFEYHJNAW-FCKHSPHMSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as retinoids. These are oxygenated derivatives of 3,7-dimethyl-1-(2,6,6-trimethylcyclohex-1-enyl)nona-1,3,5,7-tetraene and derivatives thereof. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Retinoids |
|---|
| Direct Parent | Retinoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Retinoic acid
- Diterpenoid
- Retinoid skeleton
- Medium-chain fatty acid
- Branched fatty acid
- Hydroxy fatty acid
- Methyl-branched fatty acid
- Fatty acyl
- Fatty acid
- Unsaturated fatty acid
- Carboxylic acid derivative
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Hydrocarbon derivative
- Organic oxide
- Alcohol
- Carbonyl group
- Organic oxygen compound
- Organooxygen compound
- Primary alcohol
- Aliphatic homomonocyclic compound
|
|---|
| Molecular Framework | Aliphatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Physiological effect | Not Available |
|---|
| Disposition | |
|---|
| Process | |
|---|
| Role | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Not Available |
|---|
| Predicted Molecular Properties | | Show more...
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Kovats Retention IndicesUnderivatizedDerivatized| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| all-trans-18-Hydroxyretinoic acid,1TMS,isomer #1 | CC(/C=C/C1=C(CO[Si](C)(C)C)CCCC1(C)C)=C\C=C\C(C)=C\C(=O)O | 2860.0 | Semi standard non polar | 33892256 | | all-trans-18-Hydroxyretinoic acid,1TMS,isomer #2 | CC(/C=C/C1=C(CO)CCCC1(C)C)=C\C=C\C(C)=C\C(=O)O[Si](C)(C)C | 2850.1 | Semi standard non polar | 33892256 | | all-trans-18-Hydroxyretinoic acid,2TMS,isomer #1 | CC(/C=C/C1=C(CO[Si](C)(C)C)CCCC1(C)C)=C\C=C\C(C)=C\C(=O)O[Si](C)(C)C | 2845.3 | Semi standard non polar | 33892256 | | all-trans-18-Hydroxyretinoic acid,1TBDMS,isomer #1 | CC(/C=C/C1=C(CO[Si](C)(C)C(C)(C)C)CCCC1(C)C)=C\C=C\C(C)=C\C(=O)O | 3092.7 | Semi standard non polar | 33892256 | | all-trans-18-Hydroxyretinoic acid,1TBDMS,isomer #2 | CC(/C=C/C1=C(CO)CCCC1(C)C)=C\C=C\C(C)=C\C(=O)O[Si](C)(C)C(C)(C)C | 3057.1 | Semi standard non polar | 33892256 | | all-trans-18-Hydroxyretinoic acid,2TBDMS,isomer #1 | CC(/C=C/C1=C(CO[Si](C)(C)C(C)(C)C)CCCC1(C)C)=C\C=C\C(C)=C\C(=O)O[Si](C)(C)C(C)(C)C | 3282.0 | Semi standard non polar | 33892256 |
| Show more...
|---|