| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Detected and Quantified |
|---|
| Creation Date | 2006-05-22 14:17:36 UTC |
|---|
| Update Date | 2022-03-07 02:49:12 UTC |
|---|
| HMDB ID | HMDB0002103 |
|---|
| Secondary Accession Numbers | - HMDB0062328
- HMDB02103
- HMDB62328
|
|---|
| Metabolite Identification |
|---|
| Common Name | 27-Hydroxycholesterol |
|---|
| Description | 27-Hydroxycholesterol (27-HC), also known as (25R)-cholest-5-ene-3β,26-diol or by its conventional name 26-hydroxycholesterol, is an oxygenated derivative of cholesterol and a major oxysterol in circulation (PMID: 7749852 ). 27-Hydroxycholesterol is the product of the enzyme sterol 27-hydroxylase. The enzyme is critical for the degradation of the steroid side-chain and a genetic deficiency of the enzyme leads to reduced formation of bile acids in humans. There is a correlation between 27-hydroxycholesterol and cholesterol in the circulation, and females have lower levels of 27-hydroxycholesterol than males. A strong correlation is observed between circulating levels of 27-hydroxycholesterol and cholesterol, in both healthy subjects and subjects with hypercholesterolemia and documented atherosclerosis. 27-Hydroxycholesterol is metabolized by an oxysterol 7alpha-hydroxylase in the liver. Changes in the activity of this enzyme may lead to the accumulation of 27-hydroxycholesterol in the circulation. It has been reported that patients with a genetic deficiency of oxysterol 7alpha-hydroxylase in the liver had markedly increased levels of 27-hydroxycholesterol in the circulation. However, under normal conditions and in the absence of liver or kidney disease, changes in the levels of 27-hydroxycholesterol in the circulation are likely to be caused by changes in the rate of synthesis of these steroids rather than by the rate of metabolism. There are three possible explanations for the high concentrations of 27-hydroxycholesterol found in the circulation of three subjects with atherosclerosis: (1) increased expression of sterol 27-hydroxylase owing to a genetic factor or some other factor completely unrelated to atherosclerosis, (2) the extrahepatic sterol 27-hydroxylase may be up-regulated by circulating factors (e.g. cytokines) that are directly or indirectly related to the development of atherosclerosis, and (3) the high amounts of cholesterol accumulating in macrophages in some patients with atherosclerosis may result in an increased flux of 27-hydroxycholesterol from the macrophages to the circulation. Since there is a close relation between levels of cholesterol and 27-hydroxycholesterol in the circulation, the possibility must be considered that the flux of 27-hydroxycholesterol into the brain may be part of the yet unexplained link between hypercholesterolemia and Alzheimer's disease. 27-Hydroxysterol is the most dominant oxysterol in human atheromas where it may reflect a mechanism for eliminating excessive cholesterol, and thus have a protective role. Hypercholesterolemia and chronic low-grade immunological activation are pivotal in the development of atherosclerosis. However, the interconnections between these two factors are not well known. The CD40 system, as measured by the plasma level of soluble CD40 (sCD40), is associated with cholesterol metabolism in hypercholesterolemic patients. When combined, a decreased cholesterol synthesis rate and increased levels of 27-hydroxycholesterol may be a consequence of high levels of cellular cholesterol, and therefore be related to sCD40. However, sCD40 had no significant correlation with total plasma cholesterol. This suggests that the cellular cholesterol synthesis rate and 27-hydroxycholesterol production are more importantly linked with the plasma levels of sCD40 than total cholesterol (PMID: 16081359 , 17012138 , 11504730 , 9144161 ). | Read more...
|---|
| Structure | [H][C@@]1(CC[C@@]2([H])[C@]3([H])CC=C4C[C@@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCC[C@@H](C)CO InChI=1S/C27H46O2/c1-18(17-28)6-5-7-19(2)23-10-11-24-22-9-8-20-16-21(29)12-14-26(20,3)25(22)13-15-27(23,24)4/h8,18-19,21-25,28-29H,5-7,9-17H2,1-4H3/t18-,19-,21+,22+,23-,24+,25+,26+,27-/m1/s1 |
|---|
| Synonyms | | Value | Source |
|---|
| (25R)-26-Hydroxycholesterol | ChEBI | | 5-Cholestene-3beta,27-diol | ChEBI | | Cholest-5-ene-3beta,27-diol | ChEBI | | 5-Cholestene-3b,27-diol | Generator | | 5-Cholestene-3β,27-diol | Generator | | Cholest-5-ene-3b,27-diol | Generator | | Cholest-5-ene-3β,27-diol | Generator | | (25R)-Cholest-5-ene-3b,26-diol | HMDB | | (25R)-Cholest-5-ene-3beta,26-diol | HMDB | | (3-beta)-Cholest-5-ene-3,26-diol | HMDB | | (3b,25R)-Cholest-5-ene-3,26-diol | HMDB | | (3beta,25R)-Cholest-5-ene-3,26,diol | HMDB | | 26-Hydroxycholesterol(25R) | HMDB | | Cholest-(25R)-5-en-3beta,26-diol | HMDB | | Cholest-5-ene-3-b,27-diol | HMDB | | Cholest-5-ene-3-beta,26-diol | HMDB | | Cholest-5-ene-3-beta,27-diol | HMDB | | Cholest-5-ene-3beta,26-diol | HMDB | | Cholest-5-ene-3 beta,27-diol | HMDB | | 5-Cholestene-3 beta,27-diol | HMDB | | (25R)-Cholest-5-ene-3β,26-diol | HMDB | | (3Β,25R)-cholest-5-ene-3,26-diol | HMDB | | (3beta,25R)-Cholest-5-ene-3,26-diol | HMDB | | Cholest-5-ene-3β,26-diol | HMDB | | (3Β)-cholest-5-ene-3,26-diol | HMDB | | (3beta)-Cholest-5-ene-3,26-diol | HMDB | | 26-Hydroxycholesterol | HMDB | | (3 beta,25R)-Cholest-5-ene-3,26-diol | HMDB | | 26-Hydroxycholesterol, (25R) | HMDB | | (25R)-Cholest-5-ene-3 beta,26-diol | HMDB |
| Show more...
|---|
| Chemical Formula | C27H46O2 |
|---|
| Average Molecular Weight | 402.6529 |
|---|
| Monoisotopic Molecular Weight | 402.349780716 |
|---|
| IUPAC Name | (1S,2R,5S,10S,11S,14R,15R)-14-[(2R,6R)-7-hydroxy-6-methylheptan-2-yl]-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-7-en-5-ol |
|---|
| Traditional Name | (1S,2R,5S,10S,11S,14R,15R)-14-[(2R,6R)-7-hydroxy-6-methylheptan-2-yl]-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-7-en-5-ol |
|---|
| CAS Registry Number | 20380-11-4 |
|---|
| SMILES | [H][C@@]1(CC[C@@]2([H])[C@]3([H])CC=C4C[C@@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCC[C@@H](C)CO |
|---|
| InChI Identifier | InChI=1S/C27H46O2/c1-18(17-28)6-5-7-19(2)23-10-11-24-22-9-8-20-16-21(29)12-14-26(20,3)25(22)13-15-27(23,24)4/h8,18-19,21-25,28-29H,5-7,9-17H2,1-4H3/t18-,19-,21+,22+,23-,24+,25+,26+,27-/m1/s1 |
|---|
| InChI Key | FYHRJWMENCALJY-YSQMORBQSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as dihydroxy bile acids, alcohols and derivatives. Dihydroxy bile acids, alcohols and derivatives are compounds containing or derived from a bile acid or alcohol, and which bears exactly two carboxylic acid groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Bile acids, alcohols and derivatives |
|---|
| Direct Parent | Dihydroxy bile acids, alcohols and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - 26-hydroxysteroid
- Dihydroxy bile acid, alcohol, or derivatives
- 3-hydroxy-delta-5-steroid
- 3-hydroxysteroid
- Hydroxysteroid
- 3-beta-hydroxy-delta-5-steroid
- 3-beta-hydroxysteroid
- Delta-5-steroid
- Fatty alcohol
- Fatty acyl
- Cyclic alcohol
- Secondary alcohol
- Organooxygen compound
- Alcohol
- Hydrocarbon derivative
- Organic oxygen compound
- Primary alcohol
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Physiological effect | Not Available |
|---|
| Disposition | |
|---|
| Process | |
|---|
| Role | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Not Available |
|---|
| Predicted Molecular Properties | | Show more...
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Kovats Retention IndicesUnderivatizedDerivatized| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| 27-Hydroxycholesterol,1TMS,isomer #1 | C[C@@H](CO)CCC[C@@H](C)[C@H]1CC[C@H]2[C@@H]3CC=C4C[C@@H](O[Si](C)(C)C)CC[C@]4(C)[C@H]3CC[C@@]21C | 3434.8 | Semi standard non polar | 33892256 | | 27-Hydroxycholesterol,1TMS,isomer #2 | C[C@H](CCC[C@@H](C)[C@H]1CC[C@H]2[C@@H]3CC=C4C[C@@H](O)CC[C@]4(C)[C@H]3CC[C@@]21C)CO[Si](C)(C)C | 3438.4 | Semi standard non polar | 33892256 | | 27-Hydroxycholesterol,2TMS,isomer #1 | C[C@H](CCC[C@@H](C)[C@H]1CC[C@H]2[C@@H]3CC=C4C[C@@H](O[Si](C)(C)C)CC[C@]4(C)[C@H]3CC[C@@]21C)CO[Si](C)(C)C | 3474.4 | Semi standard non polar | 33892256 | | 27-Hydroxycholesterol,1TBDMS,isomer #1 | C[C@@H](CO)CCC[C@@H](C)[C@H]1CC[C@H]2[C@@H]3CC=C4C[C@@H](O[Si](C)(C)C(C)(C)C)CC[C@]4(C)[C@H]3CC[C@@]21C | 3652.3 | Semi standard non polar | 33892256 | | 27-Hydroxycholesterol,1TBDMS,isomer #2 | C[C@H](CCC[C@@H](C)[C@H]1CC[C@H]2[C@@H]3CC=C4C[C@@H](O)CC[C@]4(C)[C@H]3CC[C@@]21C)CO[Si](C)(C)C(C)(C)C | 3697.6 | Semi standard non polar | 33892256 | | 27-Hydroxycholesterol,2TBDMS,isomer #1 | C[C@H](CCC[C@@H](C)[C@H]1CC[C@H]2[C@@H]3CC=C4C[C@@H](O[Si](C)(C)C(C)(C)C)CC[C@]4(C)[C@H]3CC[C@@]21C)CO[Si](C)(C)C(C)(C)C | 3962.0 | Semi standard non polar | 33892256 |
| Show more...
|---|
| Spectra |
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - 27-Hydroxycholesterol GC-MS (Non-derivatized) - 70eV, Positive | splash10-00du-0009000000-c409240ea9f4c08e2ba0 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 27-Hydroxycholesterol GC-MS (2 TMS) - 70eV, Positive | splash10-001i-1211490000-d6f5e80defdccceda31c | 2017-10-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 27-Hydroxycholesterol GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 27-Hydroxycholesterol 10V, Positive-QTOF | splash10-0f79-0009200000-9ee1df2377674be6691c | 2016-08-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 27-Hydroxycholesterol 20V, Positive-QTOF | splash10-00kr-2109000000-2471d899cb75de6d3684 | 2016-08-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 27-Hydroxycholesterol 40V, Positive-QTOF | splash10-0r29-3139000000-969ef6deb6437f21d9ad | 2016-08-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 27-Hydroxycholesterol 10V, Negative-QTOF | splash10-0udi-0004900000-2045d37649040dcd2d05 | 2016-08-03 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 27-Hydroxycholesterol 20V, Negative-QTOF | splash10-0ue9-0009500000-ac02d066655429b00b85 | 2016-08-03 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 27-Hydroxycholesterol 40V, Negative-QTOF | splash10-0a4r-3009000000-8b6280720e1ee0be80b4 | 2016-08-03 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 27-Hydroxycholesterol 10V, Positive-QTOF | splash10-0f79-1209500000-a16dd407d41c22cc3144 | 2021-09-23 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 27-Hydroxycholesterol 20V, Positive-QTOF | splash10-0cdr-6479100000-db657345009052fe71e1 | 2021-09-23 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 27-Hydroxycholesterol 40V, Positive-QTOF | splash10-0a4i-5910000000-84a62c4f6410b3c0eda9 | 2021-09-23 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 27-Hydroxycholesterol 10V, Negative-QTOF | splash10-0udi-0001900000-8975f0b18ceee10bde58 | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 27-Hydroxycholesterol 20V, Negative-QTOF | splash10-0udi-0004900000-459f540b58ddc4074d2a | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 27-Hydroxycholesterol 40V, Negative-QTOF | splash10-0002-0009000000-8268359a5f25a118a8c6 | 2021-09-24 | Wishart Lab | View Spectrum |
| Show more...
|---|
| Biological Properties |
|---|
| Cellular Locations | |
|---|
| Biospecimen Locations | - Blood
- Cerebrospinal Fluid (CSF)
|
|---|
| Tissue Locations | |
|---|
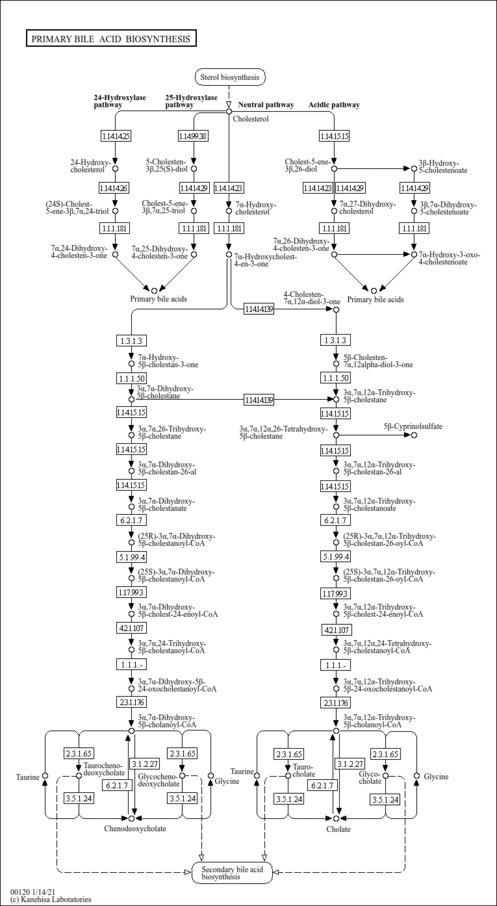
| Pathways | |
|---|
| Normal Concentrations |
|---|
| |
| Blood | Detected and Quantified | 0.538 +/- 0.04 uM | Adult (>18 years old) | Both | Normal | | details | | Blood | Detected and Quantified | 0.013 +/- 0.001 uM | Adult (>18 years old) | Both | Normal | | details | | Blood | Detected and Quantified | 0.3 +/- 0.074 uM | Adult (>18 years old) | Both | Normal | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 0.0013 +/- 0.0001 uM | Adult (>18 years old) | Both | Normal | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 0.002 +/- 0.0004 uM | Adult (>18 years old) | Both | Normal | | details |
|
|---|
| Abnormal Concentrations |
|---|
| |
| Cerebrospinal Fluid (CSF) | Detected and Quantified | 0.0026 +/- 0.0002 uM | Adult (>18 years old) | Both | Multiple sclerosis | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 0.0038 +/- 0.0003 uM | Elderly (>65 years old) | Both | Alzheimer's disease | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 0.01 +/- 0.001 uM | Adult (>18 years old) | Both | Acute or chronic demyelinating polyneuropathies | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 0.0058 +/- 0.0006 uM | Adult (>18 years old) | Both | Subarachnoid hemorrhage | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 0.006 +/- 0.0006 uM | Adult (>18 years old) | Both | Meningitis | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 0.0063 +/- 0.001 uM | Adult (>18 years old) | Both | Neuroborreliosis | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 0.0045 +/- 0.001 uM | Elderly (>65 years old) | Both | Mild cognitive impairment | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 0.003 +/- 0.0008 uM | Adult (>18 years old) | Both | Multiple sclerosis | | details |
| Show more...
|---|
| Associated Disorders and Diseases |
|---|
| Disease References | | Multiple sclerosis |
|---|
- Leoni V, Masterman T, Mousavi FS, Wretlind B, Wahlund LO, Diczfalusy U, Hillert J, Bjorkhem I: Diagnostic use of cerebral and extracerebral oxysterols. Clin Chem Lab Med. 2004 Feb;42(2):186-91. [PubMed:15061359 ]
- Leoni V, Lutjohann D, Masterman T: Levels of 7-oxocholesterol in cerebrospinal fluid are more than one thousand times lower than reported in multiple sclerosis. J Lipid Res. 2005 Feb;46(2):191-5. Epub 2004 Dec 1. [PubMed:15576852 ]
| | Alzheimer's disease |
|---|
- Leoni V, Masterman T, Mousavi FS, Wretlind B, Wahlund LO, Diczfalusy U, Hillert J, Bjorkhem I: Diagnostic use of cerebral and extracerebral oxysterols. Clin Chem Lab Med. 2004 Feb;42(2):186-91. [PubMed:15061359 ]
| | Demyelinating polyneuropathy |
|---|
- Leoni V, Masterman T, Mousavi FS, Wretlind B, Wahlund LO, Diczfalusy U, Hillert J, Bjorkhem I: Diagnostic use of cerebral and extracerebral oxysterols. Clin Chem Lab Med. 2004 Feb;42(2):186-91. [PubMed:15061359 ]
| | Subarachnoid hemorrhage |
|---|
- Leoni V, Masterman T, Mousavi FS, Wretlind B, Wahlund LO, Diczfalusy U, Hillert J, Bjorkhem I: Diagnostic use of cerebral and extracerebral oxysterols. Clin Chem Lab Med. 2004 Feb;42(2):186-91. [PubMed:15061359 ]
| | Meningitis |
|---|
- Leoni V, Masterman T, Mousavi FS, Wretlind B, Wahlund LO, Diczfalusy U, Hillert J, Bjorkhem I: Diagnostic use of cerebral and extracerebral oxysterols. Clin Chem Lab Med. 2004 Feb;42(2):186-91. [PubMed:15061359 ]
| | Neuroborreliosis |
|---|
- Leoni V, Masterman T, Mousavi FS, Wretlind B, Wahlund LO, Diczfalusy U, Hillert J, Bjorkhem I: Diagnostic use of cerebral and extracerebral oxysterols. Clin Chem Lab Med. 2004 Feb;42(2):186-91. [PubMed:15061359 ]
| | Mild cognitive impairment |
|---|
- Leoni V, Shafaati M, Salomon A, Kivipelto M, Bjorkhem I, Wahlund LO: Are the CSF levels of 24S-hydroxycholesterol a sensitive biomarker for mild cognitive impairment? Neurosci Lett. 2006 Apr 10-17;397(1-2):83-7. Epub 2006 Jan 6. [PubMed:16406316 ]
|
|
|---|
| Associated OMIM IDs | |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| Phenol Explorer Compound ID | Not Available |
|---|
| FooDB ID | FDB022846 |
|---|
| KNApSAcK ID | Not Available |
|---|
| Chemspider ID | 110495 |
|---|
| KEGG Compound ID | C06340 |
|---|
| BioCyc ID | Not Available |
|---|
| BiGG ID | 48021 |
|---|
| Wikipedia Link | 27-Hydroxycholesterol |
|---|
| METLIN ID | 6487 |
|---|
| PubChem Compound | 123976 |
|---|
| PDB ID | Not Available |
|---|
| ChEBI ID | 76591 |
|---|
| Food Biomarker Ontology | Not Available |
|---|
| VMH ID | XOL27OH |
|---|
| MarkerDB ID | MDB00000377 |
|---|
| Good Scents ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Hausheer, Frederick H.; Haridas, Kochat. Process for preparing 27-hydroxycholesterol and related derivatives. PCT Int. Appl. (1997), 38 pp. |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| General References | - Summerfield JA, Billing BH, Shackleton CH: Identification of bile acids in the serum and urine in cholestasis. Evidence for 6alpha-hydroxylation of bile acids in man. Biochem J. 1976 Feb 15;154(2):507-16. [PubMed:938463 ]
- Leoni V, Masterman T, Mousavi FS, Wretlind B, Wahlund LO, Diczfalusy U, Hillert J, Bjorkhem I: Diagnostic use of cerebral and extracerebral oxysterols. Clin Chem Lab Med. 2004 Feb;42(2):186-91. [PubMed:15061359 ]
- Burkard I, Rentsch KM, von Eckardstein A: Determination of 24S- and 27-hydroxycholesterol in plasma by high-performance liquid chromatography-mass spectrometry. J Lipid Res. 2004 Apr;45(4):776-81. Epub 2004 Jan 16. [PubMed:14729854 ]
- Fu X, Menke JG, Chen Y, Zhou G, MacNaul KL, Wright SD, Sparrow CP, Lund EG: 27-hydroxycholesterol is an endogenous ligand for liver X receptor in cholesterol-loaded cells. J Biol Chem. 2001 Oct 19;276(42):38378-87. Epub 2001 Aug 14. [PubMed:11504730 ]
- Rajagopalon I, Kok E, Cohen BI, Javitt NB: 26-Hydroxycholesterol disulfate: metabolism and excretion in the normal neonate. J Steroid Biochem. 1986 Dec;25(6):991-4. [PubMed:3795956 ]
- Li S, Pang J, Jackson EM, Wilson WK, Mott GE, Schroepfer GJ Jr: Kinetics and plasma concentrations of 26-hydroxycholesterol in baboons. Biochim Biophys Acta. 2000 May 31;1485(2-3):173-84. [PubMed:10832098 ]
- Frolov A, Zielinski SE, Crowley JR, Dudley-Rucker N, Schaffer JE, Ory DS: NPC1 and NPC2 regulate cellular cholesterol homeostasis through generation of low density lipoprotein cholesterol-derived oxysterols. J Biol Chem. 2003 Jul 11;278(28):25517-25. Epub 2003 Apr 28. [PubMed:12719428 ]
- Brown J 3rd, Theisler C, Silberman S, Magnuson D, Gottardi-Littell N, Lee JM, Yager D, Crowley J, Sambamurti K, Rahman MM, Reiss AB, Eckman CB, Wolozin B: Differential expression of cholesterol hydroxylases in Alzheimer's disease. J Biol Chem. 2004 Aug 13;279(33):34674-81. Epub 2004 May 17. [PubMed:15148325 ]
- Bellosta S, Corsini A, Bernini F, Granata A, Didoni G, Mazzotti M, Fumagalli R: 27-Hydroxycholesterol modulation of low density lipoprotein metabolism in cultured human hepatic and extrahepatic cells. FEBS Lett. 1993 Oct 11;332(1-2):115-8. [PubMed:8405424 ]
- Heverin M, Bogdanovic N, Lutjohann D, Bayer T, Pikuleva I, Bretillon L, Diczfalusy U, Winblad B, Bjorkhem I: Changes in the levels of cerebral and extracerebral sterols in the brain of patients with Alzheimer's disease. J Lipid Res. 2004 Jan;45(1):186-93. Epub 2003 Oct 1. [PubMed:14523054 ]
- Shoda J, Toll A, Axelson M, Pieper F, Wikvall K, Sjovall J: Formation of 7 alpha- and 7 beta-hydroxylated bile acid precursors from 27-hydroxycholesterol in human liver microsomes and mitochondria. Hepatology. 1993 Mar;17(3):395-403. [PubMed:8444412 ]
- Goldman M, Vlahcevic ZR, Schwartz CC, Gustafsson J, Swell L: Bile acid metabolism in cirrhosis. VIII. Quantitative evaluation of bile acid synthesis from [7 beta-3H]7 alpha-hydroxycholesterol and [G-3H]26-hydroxycholesterol. Hepatology. 1982 Jan-Feb;2(1):59-66. [PubMed:7054068 ]
- Lala DS, Syka PM, Lazarchik SB, Mangelsdorf DJ, Parker KL, Heyman RA: Activation of the orphan nuclear receptor steroidogenic factor 1 by oxysterols. Proc Natl Acad Sci U S A. 1997 May 13;94(10):4895-900. [PubMed:9144161 ]
- Norlin M, Andersson U, Bjorkhem I, Wikvall K: Oxysterol 7 alpha-hydroxylase activity by cholesterol 7 alpha-hydroxylase (CYP7A). J Biol Chem. 2000 Nov 3;275(44):34046-53. [PubMed:10882719 ]
- Babiker A, Dzeletovic S, Wiklund B, Pettersson N, Salonen J, Nyyssonen K, Eriksson M, Diczfalusy U, Bjorkhem I: Patients with atherosclerosis may have increased circulating levels of 27-hydroxycholesterol and cholestenoic acid. Scand J Clin Lab Invest. 2005;65(5):365-75. [PubMed:16081359 ]
- Luomala M, Paiva H, Laaksonen R, Thelen K, Lutjohann D, Peltonen N, Lehtimaki T: Plasma-soluble CD40 is related to cholesterol metabolism in patients with moderate hypercholesterolemia. Scand Cardiovasc J. 2006 Oct;40(5):280-4. [PubMed:17012138 ]
- Simons K, Toomre D: Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000 Oct;1(1):31-9. [PubMed:11413487 ]
- Watson AD: Thematic review series: systems biology approaches to metabolic and cardiovascular disorders. Lipidomics: a global approach to lipid analysis in biological systems. J Lipid Res. 2006 Oct;47(10):2101-11. Epub 2006 Aug 10. [PubMed:16902246 ]
- Sethi JK, Vidal-Puig AJ: Thematic review series: adipocyte biology. Adipose tissue function and plasticity orchestrate nutritional adaptation. J Lipid Res. 2007 Jun;48(6):1253-62. Epub 2007 Mar 20. [PubMed:17374880 ]
- Lingwood D, Simons K: Lipid rafts as a membrane-organizing principle. Science. 2010 Jan 1;327(5961):46-50. doi: 10.1126/science.1174621. [PubMed:20044567 ]
- Corsini A, Verri D, Raiteri M, Quarato P, Paoletti R, Fumagalli R: Effects of 26-aminocholesterol, 27-hydroxycholesterol, and 25-hydroxycholesterol on proliferation and cholesterol homeostasis in arterial myocytes. Arterioscler Thromb Vasc Biol. 1995 Mar;15(3):420-8. [PubMed:7749852 ]
- Gunstone, Frank D., John L. Harwood, and Albert J. Dijkstra (2007). The lipid handbook with CD-ROM. CRC Press.
| Show more...
|---|