| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Expected but not Quantified |
|---|
| Creation Date | 2006-08-13 07:49:56 UTC |
|---|
| Update Date | 2022-03-07 02:49:20 UTC |
|---|
| HMDB ID | HMDB0003942 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | (S)-Hydroxyhexanoyl-CoA |
|---|
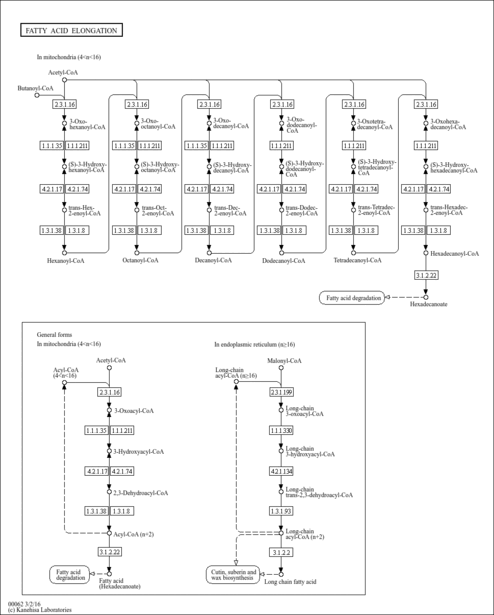
| Description | (S)-Hydroxyhexanoyl-CoA is an intermediate in fatty acid metabolism, being the substrate of the enzymes beta-hydroxyacyl-CoA dehydrogenase (EC 1.1.1.211) and 3-hydroxyacyl-CoA dehydrogenase (EC 1.1.1.35). (S)-Hydroxyhexanoyl-CoA is also an intermediate in fatty acid elongation in mitochondria, the substrate of the enzymes enoyl-CoA hydratase (EC 4.2.1.17) and long-chain-enoyl-CoA hydratase (EC 4.2.1.74) (KEGG). |
|---|
| Structure | CCC[C@H](O)CC(=O)SCCNC(=O)CCNC(=O)[C@H](O)C(C)(C)COP(O)(=O)OP(O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1OP(O)(O)=O)N1C=NC2=C1N=CN=C2N InChI=1S/C27H46N7O18P3S/c1-4-5-15(35)10-18(37)56-9-8-29-17(36)6-7-30-25(40)22(39)27(2,3)12-49-55(46,47)52-54(44,45)48-11-16-21(51-53(41,42)43)20(38)26(50-16)34-14-33-19-23(28)31-13-32-24(19)34/h13-16,20-22,26,35,38-39H,4-12H2,1-3H3,(H,29,36)(H,30,40)(H,44,45)(H,46,47)(H2,28,31,32)(H2,41,42,43)/t15-,16+,20+,21+,22-,26+/m0/s1 |
|---|
| Synonyms | | Value | Source |
|---|
| (S)-3-Hydroxycaproyl-CoA | ChEBI | | (S)-3-Hydroxycaproyl-coenzyme A | ChEBI | | 3-Hydroxyhexanoyl-coenzyme A | ChEBI | | (S)-3-Hydroxyhexanoyl-CoA | Kegg | | (S)-Hydroxyhexanoyl-CoA | ChEBI |
|
|---|
| Chemical Formula | C27H46N7O18P3S |
|---|
| Average Molecular Weight | 881.68 |
|---|
| Monoisotopic Molecular Weight | 881.183289837 |
|---|
| IUPAC Name | 4-({[({[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-hydroxy-3-(phosphonooxy)oxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy](hydroxy)phosphoryl}oxy)-2-hydroxy-N-{2-[(2-{[(3S)-3-hydroxyhexanoyl]sulfanyl}ethyl)-C-hydroxycarbonimidoyl]ethyl}-3,3-dimethylbutanimidic acid |
|---|
| Traditional Name | (S)-hydroxyhexanoyl-coa |
|---|
| CAS Registry Number | 79171-47-4 |
|---|
| SMILES | CCC[C@H](O)CC(=O)SCCNC(=O)CCNC(=O)[C@H](O)C(C)(C)COP(O)(=O)OP(O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1OP(O)(O)=O)N1C=NC2=C1N=CN=C2N |
|---|
| InChI Identifier | InChI=1S/C27H46N7O18P3S/c1-4-5-15(35)10-18(37)56-9-8-29-17(36)6-7-30-25(40)22(39)27(2,3)12-49-55(46,47)52-54(44,45)48-11-16-21(51-53(41,42)43)20(38)26(50-16)34-14-33-19-23(28)31-13-32-24(19)34/h13-16,20-22,26,35,38-39H,4-12H2,1-3H3,(H,29,36)(H,30,40)(H,44,45)(H,46,47)(H2,28,31,32)(H2,41,42,43)/t15-,16+,20+,21+,22-,26+/m0/s1 |
|---|
| InChI Key | VAAHKRMGOFIORX-IKTBLOROSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as (s)-3-hydroxyacyl coas. These are organic compounds containing a (S)-3-hydroxyl acylated coenzyme A derivative. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty acyl thioesters |
|---|
| Direct Parent | (S)-3-hydroxyacyl CoAs |
|---|
| Alternative Parents | |
|---|
| Substituents | - Coenzyme a or derivatives
- Purine ribonucleoside 3',5'-bisphosphate
- Purine ribonucleoside bisphosphate
- Purine ribonucleoside diphosphate
- Ribonucleoside 3'-phosphate
- Pentose phosphate
- Pentose-5-phosphate
- Beta amino acid or derivatives
- Glycosyl compound
- N-glycosyl compound
- 6-aminopurine
- Monosaccharide phosphate
- Organic pyrophosphate
- Pentose monosaccharide
- Imidazopyrimidine
- Purine
- Monoalkyl phosphate
- Aminopyrimidine
- Imidolactam
- N-acyl-amine
- N-substituted imidazole
- Organic phosphoric acid derivative
- Monosaccharide
- Pyrimidine
- Alkyl phosphate
- Fatty amide
- Phosphoric acid ester
- Tetrahydrofuran
- Imidazole
- Azole
- Heteroaromatic compound
- Carbothioic s-ester
- Secondary alcohol
- Thiocarboxylic acid ester
- Carboxamide group
- Secondary carboxylic acid amide
- Amino acid or derivatives
- Sulfenyl compound
- Thiocarboxylic acid or derivatives
- Organoheterocyclic compound
- Azacycle
- Oxacycle
- Carboxylic acid derivative
- Organosulfur compound
- Organic oxygen compound
- Hydrocarbon derivative
- Carbonyl group
- Organic nitrogen compound
- Primary amine
- Organopnictogen compound
- Organic oxide
- Organooxygen compound
- Organonitrogen compound
- Alcohol
- Amine
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Physiological effect | Not Available |
|---|
| Disposition | |
|---|
| Process | |
|---|
| Role | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | -3.019 | Not Available |
|
|---|
| Experimental Chromatographic Properties | Not Available |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Retention Times Underivatized| Chromatographic Method | Retention Time | Reference |
|---|
| Measured using a Waters Acquity ultraperformance liquid chromatography (UPLC) ethylene-bridged hybrid (BEH) C18 column (100 mm × 2.1 mm; 1.7 μmparticle diameter). Predicted by Afia on May 17, 2022. Predicted by Afia on May 17, 2022. | 3.74 minutes | 32390414 | | Predicted by Siyang on May 30, 2022 | 10.4335 minutes | 33406817 | | Predicted by Siyang using ReTip algorithm on June 8, 2022 | 9.54 minutes | 32390414 | | AjsUoB = Accucore 150 Amide HILIC with 10mM Ammonium Formate, 0.1% Formic Acid | 556.0 seconds | 40023050 | | Fem_Long = Waters ACQUITY UPLC HSS T3 C18 with Water:MeOH and 0.1% Formic Acid | 1200.6 seconds | 40023050 | | Fem_Lipids = Ascentis Express C18 with (60:40 water:ACN):(90:10 IPA:ACN) and 10mM NH4COOH + 0.1% Formic Acid | 169.3 seconds | 40023050 | | Life_Old = Waters ACQUITY UPLC BEH C18 with Water:(20:80 acetone:ACN) and 0.1% Formic Acid | 106.1 seconds | 40023050 | | Life_New = RP Waters ACQUITY UPLC HSS T3 C18 with Water:(30:70 MeOH:ACN) and 0.1% Formic Acid | 169.0 seconds | 40023050 | | RIKEN = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 84.5 seconds | 40023050 | | Eawag_XBridgeC18 = XBridge C18 3.5u 2.1x50 mm with Water:MeOH and 0.1% Formic Acid | 375.3 seconds | 40023050 | | BfG_NTS_RP1 =Agilent Zorbax Eclipse Plus C18 (2.1 mm x 150 mm, 3.5 um) with Water:ACN and 0.1% Formic Acid | 418.2 seconds | 40023050 | | HILIC_BDD_2 = Merck SeQuant ZIC-HILIC with ACN(0.1% formic acid):water(16 mM ammonium formate) | 949.1 seconds | 40023050 | | UniToyama_Atlantis = RP Waters Atlantis T3 (2.1 x 150 mm, 5 um) with ACN:Water and 0.1% Formic Acid | 678.9 seconds | 40023050 | | BDD_C18 = Hypersil Gold 1.9µm C18 with Water:ACN and 0.1% Formic Acid | 322.8 seconds | 40023050 | | UFZ_Phenomenex = Kinetex Core-Shell C18 2.6 um, 3.0 x 100 mm, Phenomenex with Water:MeOH and 0.1% Formic Acid | 714.6 seconds | 40023050 | | SNU_RIKEN_POS = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 222.9 seconds | 40023050 | | RPMMFDA = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 256.1 seconds | 40023050 | | MTBLS87 = Merck SeQuant ZIC-pHILIC column with ACN:Water and :ammonium carbonate | 531.6 seconds | 40023050 | | KI_GIAR_zic_HILIC_pH2_7 = Merck SeQuant ZIC-HILIC with ACN:Water and 0.1% FA | 371.3 seconds | 40023050 | | Meister zic-pHILIC pH9.3 = Merck SeQuant ZIC-pHILIC column with ACN:Water 5mM NH4Ac pH9.3 and 5mM ammonium acetate in water | 459.0 seconds | 40023050 |
Predicted Kovats Retention IndicesNot Available |
|---|
| MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - (S)-Hydroxyhexanoyl-CoA 10V, Positive-QTOF | splash10-000i-1912000130-9d32f1a7db66966548f9 | 2019-02-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - (S)-Hydroxyhexanoyl-CoA 20V, Positive-QTOF | splash10-000i-0913000000-325a72f7e906068360b8 | 2019-02-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - (S)-Hydroxyhexanoyl-CoA 40V, Positive-QTOF | splash10-000i-1911000000-391d16b13e4ae8bf53de | 2019-02-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - (S)-Hydroxyhexanoyl-CoA 10V, Negative-QTOF | splash10-001i-5911140350-4d35b969b1c6af839051 | 2019-02-23 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - (S)-Hydroxyhexanoyl-CoA 20V, Negative-QTOF | splash10-001i-3910110010-ec218967cee87cdd7488 | 2019-02-23 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - (S)-Hydroxyhexanoyl-CoA 40V, Negative-QTOF | splash10-057i-4900100000-f4b7577fa42ccd3e2dee | 2019-02-23 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - (S)-Hydroxyhexanoyl-CoA 10V, Positive-QTOF | splash10-03e9-0000000090-b8add5209e54d6b6e866 | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - (S)-Hydroxyhexanoyl-CoA 20V, Positive-QTOF | splash10-01pa-0500000290-e534ff3e17b57c33f0eb | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - (S)-Hydroxyhexanoyl-CoA 40V, Positive-QTOF | splash10-004i-0029000000-3ad72154a1670e7077a7 | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - (S)-Hydroxyhexanoyl-CoA 10V, Negative-QTOF | splash10-001i-0000000090-22f1eaad51934a88d28c | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - (S)-Hydroxyhexanoyl-CoA 20V, Negative-QTOF | splash10-00p0-7800102890-d08bf7452876ed8a38d2 | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - (S)-Hydroxyhexanoyl-CoA 40V, Negative-QTOF | splash10-002o-9302601610-9537bb62a975804266fc | 2021-09-25 | Wishart Lab | View Spectrum |
NMR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum |
|
|---|
| General References | - Dalluge JJ, Gort S, Hobson R, Selifonova O, Amore F, Gokarn R: Separation and identification of organic acid-coenzyme A thioesters using liquid chromatography/electrospray ionization-mass spectrometry. Anal Bioanal Chem. 2002 Nov;374(5):835-40. Epub 2002 Oct 8. [PubMed:12434239 ]
- Klees AG, Linder D, Buckel W: 2-Hydroxyglutaryl-CoA dehydratase from Fusobacterium nucleatum (subsp. nucleatum): an iron-sulfur flavoprotein. Arch Microbiol. 1992;158(4):294-301. [PubMed:1417419 ]
- Kim J, Hetzel M, Boiangiu CD, Buckel W: Dehydration of (R)-2-hydroxyacyl-CoA to enoyl-CoA in the fermentation of alpha-amino acids by anaerobic bacteria. FEMS Microbiol Rev. 2004 Oct;28(4):455-68. [PubMed:15374661 ]
- Hofmeister AE, Buckel W: (R)-lactyl-CoA dehydratase from Clostridium propionicum. Stereochemistry of the dehydration of (R)-2-hydroxybutyryl-CoA to crotonyl-CoA. Eur J Biochem. 1992 Jun 1;206(2):547-52. [PubMed:1597194 ]
- Kuchta RD, Hanson GR, Holmquist B, Abeles RH: Fe-S centers in lactyl-CoA dehydratase. Biochemistry. 1986 Nov 18;25(23):7301-7. [PubMed:3026450 ]
- Kuchta RD, Abeles RH: Lactate reduction in Clostridium propionicum. Purification and properties of lactyl-CoA dehydratase. J Biol Chem. 1985 Oct 25;260(24):13181-9. [PubMed:4055736 ]
- Sokatch JR: Alanine and aspartate formation during growth on valine-C14 by Pseudomonas aeruginosa. J Bacteriol. 1966 Jul;92(1):72-5. [PubMed:4957438 ]
- Megraw RE, Reeves HC, Ajl SJ: Formation of lactyl-coenzyme A and pyruvyl-coenzyme A from lactic acid by Escherichia coli. J Bacteriol. 1965 Oct;90(4):984-8. [PubMed:5321404 ]
|
|---|