| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Detected and Quantified |
|---|
| Creation Date | 2005-11-16 15:48:42 UTC |
|---|
| Update Date | 2023-02-21 17:14:40 UTC |
|---|
| HMDB ID | HMDB0000262 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Thymine |
|---|
| Description | Thymine, also known as 5-methyluracil, belongs to the class of organic compounds known as hydroxypyrimidines. These are organic compounds containing a hydroxyl group attached to a pyrimidine ring. Pyrimidine is a 6-membered ring consisting of four carbon atoms and two nitrogen centers at the 1- and 3- ring positions. Thymine was first isolated in 1893 by Albrecht Kossel and Albert Neumann from calves' thymus glands, hence its name. Thymine is one of the 4 nuelcoebases found in DNA and is essential to all life. Thymine exists in all living species, ranging from bacteria to plants to humans. Thymine combined with deoxyribose creates the nucleoside deoxythymidine (also called thymidine) which when phosphorylated to dTDP can be incorporated into DNA via DNA polymerases. Thymidine can be phosphorylated with up to three phosphoric acid groups, producing dTMP (deoxythymidine monophosphate) dTDP and/or dTTP. In RNA thymine is replaced with uracil in most cases. In DNA, thymine binds to adenine via two hydrogen bonds to assist in stabilizing the nucleic acid structures. Within humans, thymine participates in a number of enzymatic reactions. In particular, thymine and deoxyribose 1-phosphate can be biosynthesized from thymidine through its interaction with the enzyme thymidine phosphorylase. In addition, thymine can be converted into dihydrothymine; which is mediated by the enzyme dihydropyrimidine dehydrogenase [NADP(+)]. |
|---|
| Structure | InChI=1S/C5H6N2O2/c1-3-2-6-5(9)7-4(3)8/h2H,1H3,(H2,6,7,8,9) |
|---|
| Synonyms | | Value | Source |
|---|
| 2,4-Dihydroxy-5-methylpyrimidine | ChEBI | | 5-Methyl-2,4(1H,3H)-pyrimidinedione | ChEBI | | 5-Methylpyrimidine-2,4(1H,3H)-dione | ChEBI | | 5-Methyluracil | ChEBI | | T | ChEBI | | Thy | ChEBI | | Thymin | ChEBI | | 4-Hydroxy-5-methylpyrimidin-2(1H)-one | HMDB | | 5-Methyl-1,2,3,4-tetrahydropyrimidine-2,4-dione | HMDB | | 5-Methyl-2,4-dihydroxypyrimidine | HMDB | | 5-Methylpyrimidine-2,4-dione | HMDB | | 5 Methyluracil | HMDB |
|
|---|
| Chemical Formula | C5H6N2O2 |
|---|
| Average Molecular Weight | 126.1133 |
|---|
| Monoisotopic Molecular Weight | 126.042927446 |
|---|
| IUPAC Name | 5-methyl-1,2,3,4-tetrahydropyrimidine-2,4-dione |
|---|
| Traditional Name | thymine |
|---|
| CAS Registry Number | 65-71-4 |
|---|
| SMILES | CC1=CNC(=O)NC1=O |
|---|
| InChI Identifier | InChI=1S/C5H6N2O2/c1-3-2-6-5(9)7-4(3)8/h2H,1H3,(H2,6,7,8,9) |
|---|
| InChI Key | RWQNBRDOKXIBIV-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as hydroxypyrimidines. These are organic compounds containing a hydroxyl group attached to a pyrimidine ring. Pyrimidine is a 6-membered ring consisting of four carbon atoms and two nitrogen centers at the 1- and 3- ring positions. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Diazines |
|---|
| Sub Class | Pyrimidines and pyrimidine derivatives |
|---|
| Direct Parent | Hydroxypyrimidines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Hydroxypyrimidine
- Heteroaromatic compound
- Azacycle
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Physiological effect | |
|---|
| Disposition | |
|---|
| Process | |
|---|
| Role | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | 320 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 3.82 mg/mL | YALKOWSKY,SH & DANNENFELSER,RM (1992) | | LogP | -0.62 | HANSCH,C ET AL. (1995) |
|
|---|
| Experimental Chromatographic Properties | Experimental Collision Cross Sections |
|---|
| Predicted Molecular Properties | | Show more...
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Kovats Retention IndicesUnderivatizedDerivatized| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| Thymine,1TMS,isomer #1 | CC1=CN([Si](C)(C)C)C(=O)[NH]C1=O | 1549.1 | Semi standard non polar | 33892256 | | Thymine,1TMS,isomer #1 | CC1=CN([Si](C)(C)C)C(=O)[NH]C1=O | 1621.7 | Standard non polar | 33892256 | | Thymine,1TMS,isomer #1 | CC1=CN([Si](C)(C)C)C(=O)[NH]C1=O | 1986.2 | Standard polar | 33892256 | | Thymine,1TMS,isomer #2 | CC1=C[NH]C(=O)N([Si](C)(C)C)C1=O | 1530.1 | Semi standard non polar | 33892256 | | Thymine,1TMS,isomer #2 | CC1=C[NH]C(=O)N([Si](C)(C)C)C1=O | 1566.6 | Standard non polar | 33892256 | | Thymine,1TMS,isomer #2 | CC1=C[NH]C(=O)N([Si](C)(C)C)C1=O | 1979.2 | Standard polar | 33892256 | | Thymine,2TMS,isomer #1 | CC1=CN([Si](C)(C)C)C(=O)N([Si](C)(C)C)C1=O | 1733.2 | Semi standard non polar | 33892256 | | Thymine,2TMS,isomer #1 | CC1=CN([Si](C)(C)C)C(=O)N([Si](C)(C)C)C1=O | 1706.2 | Standard non polar | 33892256 | | Thymine,2TMS,isomer #1 | CC1=CN([Si](C)(C)C)C(=O)N([Si](C)(C)C)C1=O | 1809.4 | Standard polar | 33892256 | | Thymine,1TBDMS,isomer #1 | CC1=CN([Si](C)(C)C(C)(C)C)C(=O)[NH]C1=O | 1824.9 | Semi standard non polar | 33892256 | | Thymine,1TBDMS,isomer #1 | CC1=CN([Si](C)(C)C(C)(C)C)C(=O)[NH]C1=O | 1830.3 | Standard non polar | 33892256 | | Thymine,1TBDMS,isomer #1 | CC1=CN([Si](C)(C)C(C)(C)C)C(=O)[NH]C1=O | 2098.2 | Standard polar | 33892256 | | Thymine,1TBDMS,isomer #2 | CC1=C[NH]C(=O)N([Si](C)(C)C(C)(C)C)C1=O | 1723.6 | Semi standard non polar | 33892256 | | Thymine,1TBDMS,isomer #2 | CC1=C[NH]C(=O)N([Si](C)(C)C(C)(C)C)C1=O | 1788.3 | Standard non polar | 33892256 | | Thymine,1TBDMS,isomer #2 | CC1=C[NH]C(=O)N([Si](C)(C)C(C)(C)C)C1=O | 2048.3 | Standard polar | 33892256 | | Thymine,2TBDMS,isomer #1 | CC1=CN([Si](C)(C)C(C)(C)C)C(=O)N([Si](C)(C)C(C)(C)C)C1=O | 2046.2 | Semi standard non polar | 33892256 | | Thymine,2TBDMS,isomer #1 | CC1=CN([Si](C)(C)C(C)(C)C)C(=O)N([Si](C)(C)C(C)(C)C)C1=O | 2125.6 | Standard non polar | 33892256 | | Thymine,2TBDMS,isomer #1 | CC1=CN([Si](C)(C)C(C)(C)C)C(=O)N([Si](C)(C)C(C)(C)C)C1=O | 2003.8 | Standard polar | 33892256 |
| Show more...
|---|
| Spectra |
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental GC-MS | GC-MS Spectrum - Thymine GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (2 TMS) | splash10-0bt9-0960000000-50722d1f64f22b69e207 | 2014-06-16 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Thymine GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (Non-derivatized) | splash10-0bta-0940000000-ff59d40c1a93e04954ab | 2014-06-16 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Thymine GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (2 TMS) | splash10-05fr-7940000000-a0ebb190eba32aceac56 | 2014-06-16 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Thymine GC-MS (2 TMS) | splash10-0ab9-1980000000-5b95ad97f31d8176a0c6 | 2014-06-16 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Thymine EI-B (Non-derivatized) | splash10-004i-9500000000-74b6d7909610070d30d0 | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Thymine GC-EI-TOF (Non-derivatized) | splash10-0bt9-0960000000-50722d1f64f22b69e207 | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Thymine GC-EI-TOF (Non-derivatized) | splash10-0bta-0940000000-ff59d40c1a93e04954ab | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Thymine GC-EI-TOF (Non-derivatized) | splash10-05fr-7940000000-a0ebb190eba32aceac56 | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Thymine GC-MS (Non-derivatized) | splash10-0ab9-1980000000-5b95ad97f31d8176a0c6 | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Thymine GC-EI-TOF (Non-derivatized) | splash10-0bta-0930000000-a2d3bc738d3f9e60aeb7 | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Thymine GC-MS (Non-derivatized) - 70eV, Positive | splash10-004i-9500000000-33bb743c9a1a77fe4e47 | 2016-09-22 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Thymine GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Thymine GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-056r-9400000000-5dd0bc0194a302d8ee95 | 2014-09-20 | Not Available | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental LC-MS/MS | LC-MS/MS Spectrum - Thymine LC-ESI-QQ (API3000, Applied Biosystems) 10V, Negative-QTOF | splash10-004i-9100000000-1e96fdb2525aace8b29d | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Thymine LC-ESI-QQ (API3000, Applied Biosystems) 20V, Negative-QTOF | splash10-0a6r-9000000000-34edf89b8a99493ed897 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Thymine LC-ESI-QQ (API3000, Applied Biosystems) 30V, Negative-QTOF | splash10-0a4i-9000000000-7d381bc0d559ed8150e9 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Thymine LC-ESI-QQ (API3000, Applied Biosystems) 40V, Negative-QTOF | splash10-0a4i-9000000000-90f8edce6ae2b6f83347 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Thymine LC-ESI-QQ (API3000, Applied Biosystems) 50V, Negative-QTOF | splash10-0a4l-9000000000-445d07aed275008cfe2d | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Thymine LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Negative-QTOF | splash10-004i-0900000000-deb5e6830bc01a0c870e | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Thymine LC-ESI-QQ , negative-QTOF | splash10-004i-9100000000-1e96fdb2525aace8b29d | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Thymine LC-ESI-QQ , negative-QTOF | splash10-0a6r-9000000000-34edf89b8a99493ed897 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Thymine LC-ESI-QQ , negative-QTOF | splash10-0a4i-9000000000-fbf8dadffeee1dead341 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Thymine LC-ESI-QQ , negative-QTOF | splash10-0a4i-9000000000-0ee2bab9c37212ccf44e | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Thymine LC-ESI-QQ , negative-QTOF | splash10-0a4l-9000000000-599b8dcd618a9b96df4b | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Thymine LC-ESI-QTOF , negative-QTOF | splash10-004i-0900000000-deb5e6830bc01a0c870e | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Thymine 40V, Negative-QTOF | splash10-0006-9000000000-97236a7217f3f91298c3 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Thymine 10V, Negative-QTOF | splash10-0006-9200000000-5e679d49ba14eea7448e | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Thymine 20V, Negative-QTOF | splash10-0006-9000000000-90b5a25f7c3ef33245ba | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Thymine Quattro_QQQ 10V, Positive-QTOF (Annotated) | splash10-004i-0900000000-a3779faac812139c6333 | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Thymine Quattro_QQQ 25V, Positive-QTOF (Annotated) | splash10-004i-0900000000-a3779faac812139c6333 | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Thymine Quattro_QQQ 40V, Positive-QTOF (Annotated) | splash10-0udi-9200000000-0e7d9466530de47896d9 | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Thymine EI-B (HITACHI M-80) , Positive-QTOF | splash10-004i-9400000000-7ff00d5a94e12e6e1701 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Thymine LC-ESI-QQ (API3000, Applied Biosystems) 10V, Positive-QTOF | splash10-056r-8900000000-96370e30b48d3270abaa | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Thymine LC-ESI-QQ (API3000, Applied Biosystems) 20V, Positive-QTOF | splash10-0bwc-9600000000-b988fb9252c409b19c33 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Thymine LC-ESI-QQ (API3000, Applied Biosystems) 30V, Positive-QTOF | splash10-01q9-9100000000-8c82d68e9ec8594b1fff | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Thymine LC-ESI-QQ (API3000, Applied Biosystems) 40V, Positive-QTOF | splash10-001i-9000000000-f69c5d963b845f8b3cfb | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Thymine LC-ESI-QQ (API3000, Applied Biosystems) 50V, Positive-QTOF | splash10-0a4i-0900000000-3a9af2cf67d3f113fa7f | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Thymine LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Positive-QTOF | splash10-004i-0900000000-97549ff58b84351d860c | 2012-08-31 | HMDB team, MONA | View Spectrum |
NMR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Experimental 1D NMR | 1H NMR Spectrum (1D, 500 MHz, H2O, experimental) | 2012-12-04 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Experimental 1D NMR | 13C NMR Spectrum (1D, 400 MHz, H2O, experimental) | 2021-10-10 | Wishart Lab | View Spectrum | | Experimental 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 400 MHz, H2O, experimental) | 2012-12-05 | Wishart Lab | View Spectrum |
IR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M-H]-) | 2023-02-03 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M+H]+) | 2023-02-03 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M+Na]+) | 2023-02-03 | FELIX lab | View Spectrum |
| Show more...
|---|
| Biological Properties |
|---|
| Cellular Locations | |
|---|
| Biospecimen Locations | - Blood
- Cerebrospinal Fluid (CSF)
- Feces
- Saliva
- Urine
|
|---|
| Tissue Locations | - Epidermis
- Fibroblasts
- Placenta
- Prostate
|
|---|
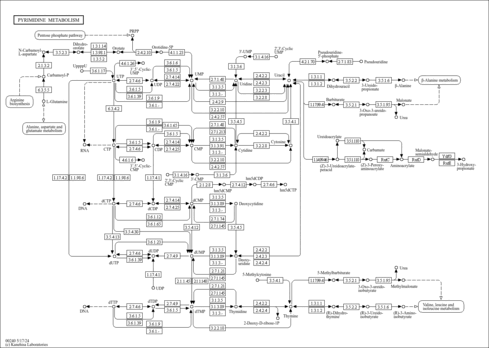
| Pathways | |
|---|
| Normal Concentrations |
|---|
| |
| Blood | Expected but not Quantified | Not Quantified | Not Available | Not Available | Normal | | details | | Blood | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | <5.0 uM | Adult (>18 years old) | Both | Normal | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 0.0 - 0.1 uM | Adult (>18 years old) | Both | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Not Specified | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Not Specified | Not Specified | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Saliva | Detected and Quantified | 5.91 +/- 11.2 uM | Adult (>18 years old) | Female | Normal | | details | | Saliva | Detected and Quantified | 1.81 +/- 2.51 uM | Adult (>18 years old) | Both | Normal | | details | | Saliva | Detected and Quantified | 8.82 +/- 13.15 uM | Adult (>18 years old) | Both | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected and Quantified | 2.89 +/- 4.53 uM | Adult (>18 years old) | Female | Normal | | details | | Saliva | Detected and Quantified | 3.51 +/- 3.10 uM | Adult (>18 years old) | Both | Normal | | details | | Saliva | Detected and Quantified | 0.655 +/- 1.44 uM | Adult (>18 years old) | Both | Normal | | details | | Urine | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Urine | Detected and Quantified | 0.10 (0.045-0.16) umol/mmol creatinine | Adult (>18 years old) | Male | Normal | | details | | Urine | Detected and Quantified | 0.16 (0.065-0.26) umol/mmol creatinine | Adult (>18 years old) | Female | Normal | | details | | Urine | Detected and Quantified | 0-9 umol/mmol creatinine | Children (1 - 18 years old) | Both | Normal | | details | | Urine | Detected and Quantified | <0.12 umol/mmol creatinine | Infant (0-1 year old) | Both | Normal | | details | | Urine | Detected and Quantified | 0.202 umol/mmol creatinine | Adult (>18 years old) | Both | Normal | | details | | Urine | Detected and Quantified | 0.791 umol/mmol creatinine | Adult (>18 years old) | Both | Normal | | details | | Urine | Detected and Quantified | 0-2 umol/mmol creatinine | Newborn (0-30 days old) | Both | Normal | | details |
| Show more...
|---|
| Abnormal Concentrations |
|---|
| |
| Blood | Detected and Quantified | 1390.0 +/- 150.0 uM | Adult (>18 years old) | Both | Solid tumors | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 0.04 - 0.2 uM | Adult (>18 years old) | Not Specified | Beta-ureidopropionase deficiency | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Colorectal cancer | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Colorectal Cancer | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Colorectal cancer | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Periodontal Probing Depth | | details | | Saliva | Detected and Quantified | 2.61 +/- 2.39 uM | Adult (>18 years old) | Both | Temporomandibular joint disorder (TMD) | | details | | Urine | Detected and Quantified | 343.024 umol/mmol creatinine | Adult (>18 years old) | Female | Dihydropyrimidine dehydrogenase (DPD) deficiency | | details | | Urine | Detected and Quantified | 468.0684 umol/mmol creatinine | Children (1 - 13 years old) | Male | Dihydropyrimidine dehydrogenase (DPD) deficiency | | details |
| Show more...
|---|
| Associated Disorders and Diseases |
|---|
| Disease References | | Thymidine treatment |
|---|
- Leyva A, Schornagel JH, Kraal I, Wadman SK, Pinedo HM: Clinical and biochemical studies of high-dose thymidine treatment in patients with solid tumors. J Cancer Res Clin Oncol. 1984;107(3):211-6. [PubMed:6736109 ]
| | Beta-ureidopropionase deficiency |
|---|
- van Kuilenburg AB, Meinsma R, Beke E, Assmann B, Ribes A, Lorente I, Busch R, Mayatepek E, Abeling NG, van Cruchten A, Stroomer AE, van Lenthe H, Zoetekouw L, Kulik W, Hoffmann GF, Voit T, Wevers RA, Rutsch F, van Gennip AH: beta-Ureidopropionase deficiency: an inborn error of pyrimidine degradation associated with neurological abnormalities. Hum Mol Genet. 2004 Nov 15;13(22):2793-801. Epub 2004 Sep 22. [PubMed:15385443 ]
| | Colorectal cancer |
|---|
- Brown DG, Rao S, Weir TL, O'Malia J, Bazan M, Brown RJ, Ryan EP: Metabolomics and metabolic pathway networks from human colorectal cancers, adjacent mucosa, and stool. Cancer Metab. 2016 Jun 6;4:11. doi: 10.1186/s40170-016-0151-y. eCollection 2016. [PubMed:27275383 ]
- Sinha R, Ahn J, Sampson JN, Shi J, Yu G, Xiong X, Hayes RB, Goedert JJ: Fecal Microbiota, Fecal Metabolome, and Colorectal Cancer Interrelations. PLoS One. 2016 Mar 25;11(3):e0152126. doi: 10.1371/journal.pone.0152126. eCollection 2016. [PubMed:27015276 ]
- Goedert JJ, Sampson JN, Moore SC, Xiao Q, Xiong X, Hayes RB, Ahn J, Shi J, Sinha R: Fecal metabolomics: assay performance and association with colorectal cancer. Carcinogenesis. 2014 Sep;35(9):2089-96. doi: 10.1093/carcin/bgu131. Epub 2014 Jul 18. [PubMed:25037050 ]
| | Temporomandibular joint disorder |
|---|
- (). Sugimoto et al. (2013) Physiological and environmental parameters associated with mass spectrometry-based salivary metabolomic profiles. . .
| | Periodontal Probing Depth |
|---|
- Liebsch C, Pitchika V, Pink C, Samietz S, Kastenmuller G, Artati A, Suhre K, Adamski J, Nauck M, Volzke H, Friedrich N, Kocher T, Holtfreter B, Pietzner M: The Saliva Metabolome in Association to Oral Health Status. J Dent Res. 2019 Jun;98(6):642-651. doi: 10.1177/0022034519842853. Epub 2019 Apr 26. [PubMed:31026179 ]
| | Dihydropyrimidine dehydrogenase deficiency |
|---|
- Brockstedt M, Jakobs C, Smit LM, van Gennip AH, Berger R: A new case of dihydropyrimidine dehydrogenase deficiency. J Inherit Metab Dis. 1990;13(1):121-4. [PubMed:2109146 ]
- G.Frauendienst-Egger, Friedrich K. Trefz (2017). MetaGene: Metabolic & Genetic Information Center (MIC: http://www.metagene.de). METAGENE consortium.
|
|
|---|
| Associated OMIM IDs | - 613161 (Beta-ureidopropionase deficiency)
- 114500 (Colorectal cancer)
- 274270 (Dihydropyrimidine dehydrogenase deficiency)
|
|---|
| External Links |
|---|
| DrugBank ID | DB03462 |
|---|
| Phenol Explorer Compound ID | Not Available |
|---|
| FooDB ID | FDB021922 |
|---|
| KNApSAcK ID | C00001511 |
|---|
| Chemspider ID | 1103 |
|---|
| KEGG Compound ID | C00178 |
|---|
| BioCyc ID | THYMINE |
|---|
| BiGG ID | 34151 |
|---|
| Wikipedia Link | Thymine |
|---|
| METLIN ID | 290 |
|---|
| PubChem Compound | 1135 |
|---|
| PDB ID | Not Available |
|---|
| ChEBI ID | 17821 |
|---|
| Food Biomarker Ontology | Not Available |
|---|
| VMH ID | THYM |
|---|
| MarkerDB ID | MDB00000127 |
|---|
| Good Scents ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Zhang, Shi-Ying; Wu, Da-Jun; Zhang, Yan-Ping. Synthesis of thymine. Zhongguo Yiyao Gongye Zazhi (1999), 30(7), 325. |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| General References | - Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, Laxman B, Mehra R, Lonigro RJ, Li Y, Nyati MK, Ahsan A, Kalyana-Sundaram S, Han B, Cao X, Byun J, Omenn GS, Ghosh D, Pennathur S, Alexander DC, Berger A, Shuster JR, Wei JT, Varambally S, Beecher C, Chinnaiyan AM: Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009 Feb 12;457(7231):910-4. doi: 10.1038/nature07762. [PubMed:19212411 ]
- Eells JT, Spector R: Purine and pyrimidine base and nucleoside concentrations in human cerebrospinal fluid and plasma. Neurochem Res. 1983 Nov;8(11):1451-7. [PubMed:6656991 ]
- Hofmann U, Schwab M, Seefried S, Marx C, Zanger UM, Eichelbaum M, Murdter TE: Sensitive method for the quantification of urinary pyrimidine metabolites in healthy adults by gas chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2003 Jul 5;791(1-2):371-80. [PubMed:12798197 ]
- van Lenthe H, van Kuilenburg AB, Ito T, Bootsma AH, van Cruchten A, Wada Y, van Gennip AH: Defects in pyrimidine degradation identified by HPLC-electrospray tandem mass spectrometry of urine specimens or urine-soaked filter paper strips. Clin Chem. 2000 Dec;46(12):1916-22. [PubMed:11106323 ]
- Allgayer H, Kolb M, Stuber V, Kruis W: Effects of bile acids on base hydroxylation in a model of human colonic mucosal DNA. Cancer Detect Prev. 2002;26(1):85-9. [PubMed:12088208 ]
- Goukassian D, Gad F, Yaar M, Eller MS, Nehal US, Gilchrest BA: Mechanisms and implications of the age-associated decrease in DNA repair capacity. FASEB J. 2000 Jul;14(10):1325-34. [PubMed:10877825 ]
- Wassberg C, Backvall H, Diffey B, Ponten F, Berne B: Enhanced epidermal ultraviolet responses in chronically sun-exposed skin are dependent on previous sun exposure. Acta Derm Venereol. 2003;83(4):254-61. [PubMed:12926795 ]
- Schilsky RL, O'Laughlin K, Ratain MJ: Phase I clinical and pharmacological study of thymidine (NSC 21548) and cis-diamminedichloroplatinum(II) in patients with advanced cancer. Cancer Res. 1986 Aug;46(8):4184-8. [PubMed:3731086 ]
- Maskell R, Okubadejo OA, Payne RH, Pead L: Human infections with thymine-requiring bacteria. J Med Microbiol. 1978 Feb;11(1):33-45. [PubMed:621731 ]
- Ling G, Chadwick CA, Berne B, Potten CS, Ponten J, Ponten F: Epidermal p53 response and repair of thymine dimers in human skin after a single dose of ultraviolet radiation: effects of photoprotection. Acta Derm Venereol. 2001 May;81(2):81-6. [PubMed:11501666 ]
- Young AR, Sheehan JM, Chadwick CA, Potten CS: Protection by ultraviolet A and B sunscreens against in situ dipyrimidine photolesions in human epidermis is comparable to protection against sunburn. J Invest Dermatol. 2000 Jul;115(1):37-41. [PubMed:10886505 ]
- Maskell R, Okubadejo OA, Payne RH: Thymine-requiring bacteria associated with co-trimoxazole therapy. Lancet. 1976 Apr 17;1(7964):834-5. [PubMed:56651 ]
- Courdavault S, Baudouin C, Sauvaigo S, Mouret S, Candeias S, Charveron M, Favier A, Cadet J, Douki T: Unrepaired cyclobutane pyrimidine dimers do not prevent proliferation of UV-B-irradiated cultured human fibroblasts. Photochem Photobiol. 2004 Feb;79(2):145-51. [PubMed:15068027 ]
- Alsarra IA, Alarifi MN: Validated liquid chromatographic determination of 5-fluorouracil in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2004 May 25;804(2):435-9. [PubMed:15081940 ]
- Castro-Gago M, Camina F, Lojo S, Rodriguez-Segade S, Rodriguez-Nunez A: Concentrations of purine nucleotides and purine and pyrimidine bases in cerebrospinal fluid of neurologically healthy children. Eur J Clin Chem Clin Biochem. 1992 Nov;30(11):761-5. [PubMed:1489848 ]
- Placzek M, Gaube S, Kerkmann U, Gilbertz KP, Herzinger T, Haen E, Przybilla B: Ultraviolet B-induced DNA damage in human epidermis is modified by the antioxidants ascorbic acid and D-alpha-tocopherol. J Invest Dermatol. 2005 Feb;124(2):304-7. [PubMed:15675947 ]
- Thienpont LM, Van Landuyt KG, Stockl D, Saeyens W, De Keukeleire D, De Leenheer AP: Evaluation of 2-iminoimidazolidin-4-one and thymine as respective internal standards for normal-phase and reversed-phase high-performance liquid chromatographic determination of creatinine in human serum. J Chromatogr B Biomed Appl. 1995 Mar 10;665(1):63-9. [PubMed:7795802 ]
- Rodriguez Ortner E, Hayes RB, Weissfeld J, Gelmann EP: Effect of homeodomain protein NKX3.1 R52C polymorphism on prostate gland size. Urology. 2006 Feb;67(2):311-5. Epub 2006 Jan 25. [PubMed:16442598 ]
- Antille C, Tran C, Sorg O, Carraux P, Didierjean L, Saurat JH: Vitamin A exerts a photoprotective action in skin by absorbing ultraviolet B radiation. J Invest Dermatol. 2003 Nov;121(5):1163-7. [PubMed:14708621 ]
- Elshenawy S, Pinney SE, Stuart T, Doulias PT, Zura G, Parry S, Elovitz MA, Bennett MJ, Bansal A, Strauss JF 3rd, Ischiropoulos H, Simmons RA: The Metabolomic Signature of the Placenta in Spontaneous Preterm Birth. Int J Mol Sci. 2020 Feb 4;21(3). pii: ijms21031043. doi: 10.3390/ijms21031043. [PubMed:32033212 ]
| Show more...
|---|