| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Expected but not Quantified |
|---|
| Creation Date | 2005-11-16 15:48:42 UTC |
|---|
| Update Date | 2022-03-07 02:49:08 UTC |
|---|
| HMDB ID | HMDB0001022 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Succinyl-CoA |
|---|
| Description | Succinyl-CoA is an important intermediate in the citric acid cycle, where it is synthesized from α-Ketoglutarate by α-ketoglutarate dehydrogenase (EC 1.2.4.2) through decarboxylation, and is converted into succinate through the hydrolytic release of coenzyme A by succinyl-CoA synthetase (EC 6.2.1.5). Succinyl-CoA may be an end product of peroxisomal beta-oxidation of dicarboxylic fatty acids; the identification of an apparently specific succinyl-CoA thioesterase (ACOT4, EC 3.1.2.3, hydrolyzes succinyl-CoA) in peroxisomes strongly suggests that succinyl-CoA is formed in peroxisomes. Acyl-CoA thioesterases (ACOTs) are a family of enzymes that catalyze the hydrolysis of the CoA esters of various lipids to the free acids and coenzyme A, thereby regulating levels of these compounds. (PMID: 16141203 ). |
|---|
| Structure | CC(C)(COP(O)(=O)OP(O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1OP(O)(O)=O)N1C=NC2=C1N=CN=C2N)[C@@H](O)C(=O)NCCC(=O)NCCSC(=O)CCC(O)=O InChI=1S/C25H40N7O19P3S/c1-25(2,20(38)23(39)28-6-5-14(33)27-7-8-55-16(36)4-3-15(34)35)10-48-54(45,46)51-53(43,44)47-9-13-19(50-52(40,41)42)18(37)24(49-13)32-12-31-17-21(26)29-11-30-22(17)32/h11-13,18-20,24,37-38H,3-10H2,1-2H3,(H,27,33)(H,28,39)(H,34,35)(H,43,44)(H,45,46)(H2,26,29,30)(H2,40,41,42)/t13-,18-,19-,20+,24-/m1/s1 |
|---|
| Synonyms | | Value | Source |
|---|
| coenzyme A, S-(Hydrogen butanedioate) | ChEBI | | S-(3-Carboxy-propionyl)-CoA | ChEBI | | S-(3-Carboxy-propionyl)-coenzym-a | ChEBI | | S-(3-Carboxypropionyl)-coenzyme A | ChEBI | | S-(Hydrogen succinyl)-CoA | ChEBI | | S-(Hydrogen succinyl)coenzyme A | ChEBI | | Succinyl coenzyme A | ChEBI | | coenzyme A, S-(Hydrogen butanedioic acid) | Generator | | Succinyl-coenzyme A | ChEBI | | S-Succinoylcoenzyme A | HMDB | | Succinyl CoA | HMDB | | Succinyl-CoA | HMDB |
|
|---|
| Chemical Formula | C25H40N7O19P3S |
|---|
| Average Molecular Weight | 867.607 |
|---|
| Monoisotopic Molecular Weight | 867.131252359 |
|---|
| IUPAC Name | 4-[(2-{3-[(2R)-3-[({[({[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-hydroxy-3-(phosphonooxy)oxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy](hydroxy)phosphoryl}oxy)methyl]-2-hydroxy-3-methylbutanamido]propanamido}ethyl)sulfanyl]-4-oxobutanoic acid |
|---|
| Traditional Name | succinyl-coa |
|---|
| CAS Registry Number | 604-98-8 |
|---|
| SMILES | CC(C)(COP(O)(=O)OP(O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1OP(O)(O)=O)N1C=NC2=C1N=CN=C2N)[C@@H](O)C(=O)NCCC(=O)NCCSC(=O)CCC(O)=O |
|---|
| InChI Identifier | InChI=1S/C25H40N7O19P3S/c1-25(2,20(38)23(39)28-6-5-14(33)27-7-8-55-16(36)4-3-15(34)35)10-48-54(45,46)51-53(43,44)47-9-13-19(50-52(40,41)42)18(37)24(49-13)32-12-31-17-21(26)29-11-30-22(17)32/h11-13,18-20,24,37-38H,3-10H2,1-2H3,(H,27,33)(H,28,39)(H,34,35)(H,43,44)(H,45,46)(H2,26,29,30)(H2,40,41,42)/t13-,18-,19-,20+,24-/m1/s1 |
|---|
| InChI Key | VNOYUJKHFWYWIR-ITIYDSSPSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as acyl coas. These are organic compounds containing a coenzyme A substructure linked to an acyl chain. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty acyl thioesters |
|---|
| Direct Parent | Acyl CoAs |
|---|
| Alternative Parents | |
|---|
| Substituents | - Coenzyme a or derivatives
- Purine ribonucleoside diphosphate
- Purine ribonucleoside bisphosphate
- Purine ribonucleoside 3',5'-bisphosphate
- Ribonucleoside 3'-phosphate
- Pentose-5-phosphate
- Pentose phosphate
- Glycosyl compound
- N-glycosyl compound
- Organic pyrophosphate
- Pentose monosaccharide
- 6-aminopurine
- Monosaccharide phosphate
- Purine
- Imidazopyrimidine
- Hydroxy fatty acid
- Thia fatty acid
- Aminopyrimidine
- Monoalkyl phosphate
- Monosaccharide
- N-substituted imidazole
- Organic phosphoric acid derivative
- Imidolactam
- Phosphoric acid ester
- Alkyl phosphate
- Pyrimidine
- Tetrahydrofuran
- Azole
- Imidazole
- Heteroaromatic compound
- Thiocarboxylic acid ester
- Secondary alcohol
- Carbothioic s-ester
- Amino acid or derivatives
- Amino acid
- Thiocarboxylic acid or derivatives
- Propargyl-type 1,3-dipolar organic compound
- Organic 1,3-dipolar compound
- Sulfenyl compound
- Carboximidic acid
- Carboximidic acid derivative
- Carboxylic acid derivative
- Carboxylic acid
- Organoheterocyclic compound
- Monocarboxylic acid or derivatives
- Oxacycle
- Azacycle
- Organopnictogen compound
- Organic oxide
- Organic oxygen compound
- Hydrocarbon derivative
- Organosulfur compound
- Organooxygen compound
- Primary amine
- Organic nitrogen compound
- Alcohol
- Amine
- Carbonyl group
- Organonitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Not Available | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Not Available |
|---|
| Predicted Molecular Properties | | Show more...
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Kovats Retention IndicesUnderivatized | Show more...
|---|
| Spectra |
|---|
| MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Succinyl-CoA 10V, Positive-QTOF | splash10-000i-1912000130-5d37f85b68294f1bcd7b | 2015-09-14 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Succinyl-CoA 20V, Positive-QTOF | splash10-000i-0913000000-5f3ce9a55c6b75d06990 | 2015-09-14 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Succinyl-CoA 40V, Positive-QTOF | splash10-000i-2911000000-d4965f3bcf572b49e153 | 2015-09-14 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Succinyl-CoA 10V, Negative-QTOF | splash10-017j-8921150580-9a135870fabc65b05d2e | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Succinyl-CoA 20V, Negative-QTOF | splash10-003s-4910010010-1b02ce20692d0ded522e | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Succinyl-CoA 40V, Negative-QTOF | splash10-057i-6900100000-44f1bfaeb10b1463a0bd | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Succinyl-CoA 10V, Negative-QTOF | splash10-014i-0000000090-bb5a570be808ea8a3dd8 | 2021-09-23 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Succinyl-CoA 20V, Negative-QTOF | splash10-00kg-9100001430-6a56696bfa6b84fc9826 | 2021-09-23 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Succinyl-CoA 40V, Negative-QTOF | splash10-016r-8102403910-3e8c6747e8bdbda6db09 | 2021-09-23 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Succinyl-CoA 10V, Positive-QTOF | splash10-014r-0300000090-ce1ea17de736a3128d73 | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Succinyl-CoA 20V, Positive-QTOF | splash10-000i-0901000020-307a08ff7ab07e0b6165 | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Succinyl-CoA 40V, Positive-QTOF | splash10-03di-1119000000-859564ca28518883d359 | 2021-09-24 | Wishart Lab | View Spectrum |
NMR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum |
| Show more...
|---|
| Biological Properties |
|---|
| Cellular Locations | - Extracellular
- Membrane
- Mitochondria
|
|---|
| Biospecimen Locations | Not Available |
|---|
| Tissue Locations | |
|---|
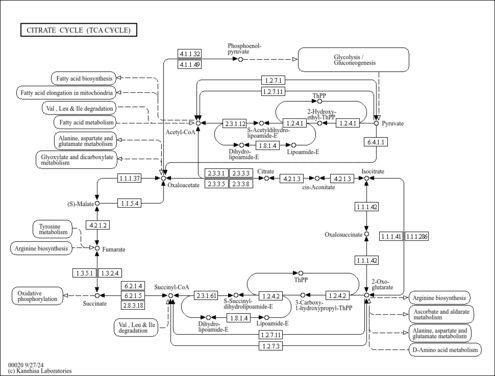
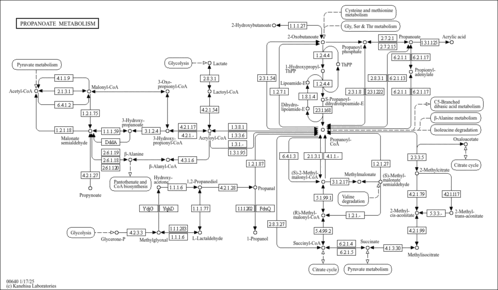
| Pathways | |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| Associated Disorders and Diseases |
|---|
| Disease References | None |
|---|
| Associated OMIM IDs | None |
|---|
| External Links |
|---|
| DrugBank ID | DB03699 |
|---|
| Phenol Explorer Compound ID | Not Available |
|---|
| FooDB ID | FDB022375 |
|---|
| KNApSAcK ID | C00019546 |
|---|
| Chemspider ID | 83179 |
|---|
| KEGG Compound ID | C00091 |
|---|
| BioCyc ID | SUC-COA |
|---|
| BiGG ID | Not Available |
|---|
| Wikipedia Link | Succinyl-CoA |
|---|
| METLIN ID | Not Available |
|---|
| PubChem Compound | 92133 |
|---|
| PDB ID | Not Available |
|---|
| ChEBI ID | 15380 |
|---|
| Food Biomarker Ontology | Not Available |
|---|
| VMH ID | SUCCOA |
|---|
| MarkerDB ID | Not Available |
|---|
| Good Scents ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Wollemann, M. Mechanism of the succinyl-coenzyme A synthesis in brain extracts. Acta Physiologica Academiae Scientiarum Hungaricae (1959), 16 153-4. |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| General References | - Tanaka H, Kohroki J, Iguchi N, Onishi M, Nishimune Y: Cloning and characterization of a human orthologue of testis-specific succinyl CoA: 3-oxo acid CoA transferase (Scot-t) cDNA. Mol Hum Reprod. 2002 Jan;8(1):16-23. [PubMed:11756565 ]
- Elpeleg O, Miller C, Hershkovitz E, Bitner-Glindzicz M, Bondi-Rubinstein G, Rahman S, Pagnamenta A, Eshhar S, Saada A: Deficiency of the ADP-forming succinyl-CoA synthase activity is associated with encephalomyopathy and mitochondrial DNA depletion. Am J Hum Genet. 2005 Jun;76(6):1081-6. Epub 2005 Apr 22. [PubMed:15877282 ]
- Westin MA, Hunt MC, Alexson SE: The identification of a succinyl-CoA thioesterase suggests a novel pathway for succinate production in peroxisomes. J Biol Chem. 2005 Nov 18;280(46):38125-32. Epub 2005 Aug 31. [PubMed:16141203 ]
|
|---|