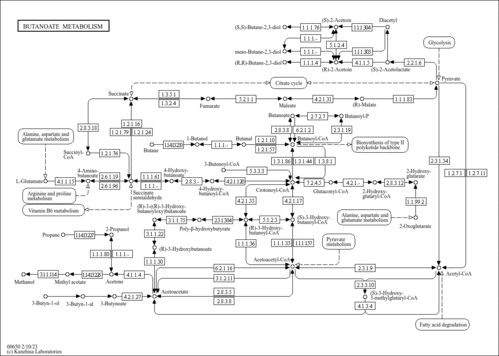
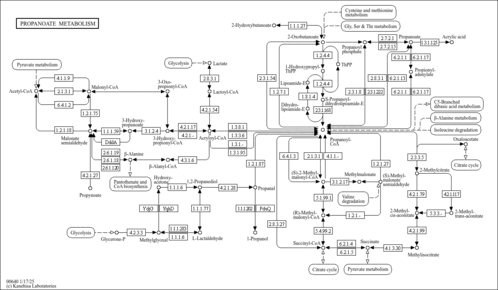
| MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental LC-MS/MS | LC-MS/MS Spectrum - Acetoacetyl-CoA n/a 59V, negative-QTOF | splash10-000i-0004009000-9559fa356f1bae03b7ca | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Acetoacetyl-CoA n/a 59V, negative-QTOF | splash10-0a4i-0269300000-d5574d3fd84812c0057d | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Acetoacetyl-CoA n/a 59V, negative-QTOF | splash10-05n1-0009600000-a29b94fc4d454c655bab | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Acetoacetyl-CoA n/a 59V, negative-QTOF | splash10-000i-0001209000-21979fdba010c3871c5a | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Acetoacetyl-CoA Orbitrap 42V, negative-QTOF | splash10-0udi-0000000090-aa1f97ad009df4ad9439 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Acetoacetyl-CoA Orbitrap 57V, negative-QTOF | splash10-0udi-0000100490-97b56edeb43a5d82ba85 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Acetoacetyl-CoA Orbitrap 71V, negative-QTOF | splash10-014i-0000910830-9d7a9bb5d2817f98fbf1 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Acetoacetyl-CoA Orbitrap 93V, negative-QTOF | splash10-0ar9-0101901000-ee3c1d5ebb3142f3c5af | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Acetoacetyl-CoA Orbitrap 115V, negative-QTOF | splash10-0a6r-5524900000-2d745cfa452462059e2c | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Acetoacetyl-CoA Orbitrap 136V, negative-QTOF | splash10-056r-9513200000-037d88669e973d0dff1c | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Acetoacetyl-CoA Orbitrap 158V, negative-QTOF | splash10-004i-9400000000-fca9f3020e4e007e8164 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Acetoacetyl-CoA Orbitrap 187V, negative-QTOF | splash10-004i-9300000000-13ad94b2682d99b6234d | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Acetoacetyl-CoA Orbitrap 216V, negative-QTOF | splash10-004i-9200000000-32dfa0b8d343e2eb7b7b | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Acetoacetyl-CoA Orbitrap 260V, negative-QTOF | splash10-004i-9100000000-6461ef6280ce0db781b2 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Acetoacetyl-CoA n/a 59V, negative-QTOF | splash10-014i-0000000900-727bb0c3c925e7bbeba4 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Acetoacetyl-CoA n/a 59V, negative-QTOF | splash10-00di-0190000000-0e093fb5398057b2093d | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Acetoacetyl-CoA n/a 59V, negative-QTOF | splash10-0a4i-0900000000-b4c90f8c858e783159b8 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Acetoacetyl-CoA n/a 59V, negative-QTOF | splash10-0udl-0018900000-91aaf6888a10aac16f57 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Acetoacetyl-CoA n/a 59V, negative-QTOF | splash10-00xr-0092000000-2dd3c44dfb7a39ee9a59 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Acetoacetyl-CoA n/a 59V, negative-QTOF | splash10-0670-0000904200-f3bcbcab3db17ae6a02a | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Acetoacetyl-CoA n/a 59V, negative-QTOF | splash10-00di-0190000000-3a982abdcfdcfe6b0420 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Acetoacetyl-CoA n/a 59V, negative-QTOF | splash10-0udr-0027900000-0d41e18ae4bea1b5b7eb | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Acetoacetyl-CoA n/a 59V, positive-QTOF | splash10-0002-6900000000-959cba779002108c94cc | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Acetoacetyl-CoA n/a 59V, positive-QTOF | splash10-00di-9000000000-9fd58d8b09e3b71e5e6f | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Acetoacetyl-CoA n/a 59V, positive-QTOF | splash10-03di-0190000000-aa6e0456b3c35ec78a6a | 2020-07-22 | HMDB team, MONA | View Spectrum |
NMR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum |
| Show more...
|---|