| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Detected and Quantified |
|---|
| Creation Date | 2006-05-22 15:12:24 UTC |
|---|
| Update Date | 2023-02-21 17:16:30 UTC |
|---|
| HMDB ID | HMDB0002991 |
|---|
| Secondary Accession Numbers | - HMDB0060150
- HMDB02991
- HMDB60150
|
|---|
| Metabolite Identification |
|---|
| Common Name | Cysteamine |
|---|
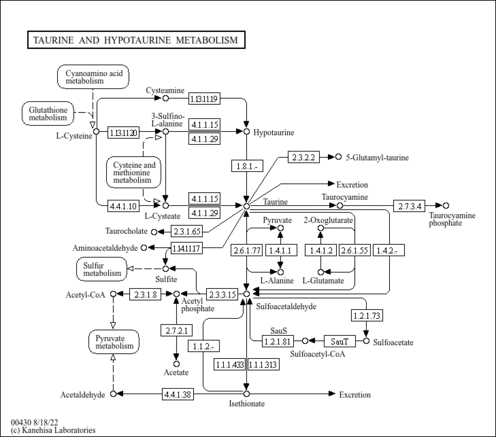
| Description | Cysteamine is a product of the constitutive degradation of coenzyme A, a process that occurs in all tissues, although some tissues such as brain and heart may have exceptionally high coenzyme A turnover rates. Cysteamine has only one known function, and that is as a precursor for the formation of hypotaurine, which is subsequently oxidized to taurine. The rate of cysteamine production as a result of coenzyme A breakdown is not well understood but it is clear that cysteamine levels are not as dramatically affected by dietary habits as are cysteine levels. Cysteamine is generated from hypotaurine by cysteamine dioxygenase (EC:1.13.11.19), an enzyme that was recently identified in mammals (PMID:17581819 ). Cysteamine is the simplest stable aminothiol found in the body. It is used in the treatment of disorders of cystine excretion. Cysteamine cleaves the disulfide bond with cysteine to produce molecules that can escape the metabolic defect in cystinosis and cystinuria. Cyst(e)amine may also serve as an endogenous regulator of immune system activity as well as a potential therapeutic agent for the treatment of Huntington disease. Cysteamine is also used as a radiation-protective agent that oxidizes in air to form cystamine. It can be given intravenously or orally to treat radiation sickness. |
|---|
| Structure | InChI=1S/C2H7NS/c3-1-2-4/h4H,1-3H2 |
|---|
| Synonyms | | Value | Source |
|---|
| 2-Amino-1-ethanethiol | ChEBI | | 2-AMINO-ethanethiol | ChEBI | | 2-Aminoethanethiol | ChEBI | | beta-Aminoethanethiol | ChEBI | | beta-Aminoethylthiol | ChEBI | | beta-MEA | ChEBI | | beta-Mercaptoethylamine | ChEBI | | MEA | ChEBI | | Mercaptamina | ChEBI | | Mercaptamine | ChEBI | | Mercaptaminum | ChEBI | | Thioethanolamine | ChEBI | | b-Aminoethanethiol | Generator | | Β-aminoethanethiol | Generator | | b-Aminoethylthiol | Generator | | Β-aminoethylthiol | Generator | | b-MEA | Generator | | Β-mea | Generator | | b-Mercaptoethylamine | Generator | | Β-mercaptoethylamine | Generator | | (2-Mercaptoethyl)amine | HMDB | | 2-Aminoethyl mercaptan | HMDB | | 2-Mercaptoethanamine | HMDB | | Aminoethyl mercaptan | HMDB | | Becaptan | HMDB | | CASH | HMDB | | Cisteamina | HMDB | | Cysteamide | HMDB | | Cysteamin | HMDB | | Cysteaminium | HMDB | | Cysteinamine | HMDB | | Decarboxycysteine | HMDB | | Ethanethiolamine | HMDB | | Lambraten | HMDB | | Lambratene | HMDB | | Mecramine | HMDB | | Mercamin | HMDB | | Mercamine | HMDB | | Mercaptamin | HMDB | | Merkamin | HMDB | | Riacon | HMDB | | 35S-Labeled cysteamine | HMDB | | Cystagon | HMDB | | Cysteamine hydrobromide | HMDB | | Hydrochloride, cysteamine | HMDB | | Tosylate, cysteamine | HMDB | | Cysteamine dihydrochloride | HMDB | | Cysteamine hydrochloride | HMDB | | Cysteamine maleate (1:1) | HMDB | | Cysteamine tosylate | HMDB | | Mercaptoethylamine | HMDB | | 2 Aminoethanethiol | HMDB | | Cysteamine bitartrate | HMDB | | Cysteamine tartrate | HMDB | | Cysteamine tartrate (1:1) | HMDB | | Hydrobromide, cysteamine | HMDB | | Tartrate, cysteamine | HMDB | | beta Mercaptoethylamine | HMDB | | Bitartrate, cysteamine | HMDB | | Cysteamine, 35S labeled | HMDB | | Cysteamine, 35S-labeled | HMDB | | Dihydrochloride, cysteamine | HMDB |
| Show more...
|---|
| Chemical Formula | C2H7NS |
|---|
| Average Molecular Weight | 77.149 |
|---|
| Monoisotopic Molecular Weight | 77.029919919 |
|---|
| IUPAC Name | 2-aminoethane-1-thiol |
|---|
| Traditional Name | cysteamine |
|---|
| CAS Registry Number | 60-23-1 |
|---|
| SMILES | NCCS |
|---|
| InChI Identifier | InChI=1S/C2H7NS/c3-1-2-4/h4H,1-3H2 |
|---|
| InChI Key | UFULAYFCSOUIOV-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as alkylthiols. These are organic compounds containing the thiol functional group linked to an alkyl chain. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organosulfur compounds |
|---|
| Class | Thiols |
|---|
| Sub Class | Alkylthiols |
|---|
| Direct Parent | Alkylthiols |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alkylthiol
- Organic nitrogen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Primary amine
- Organonitrogen compound
- Primary aliphatic amine
- Amine
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Physiological effect | Not Available |
|---|
| Disposition | |
|---|
| Process | |
|---|
| Role | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | 98 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Not Available |
|---|
| Predicted Molecular Properties | | Show more...
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Kovats Retention IndicesUnderivatized| Metabolite | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| Cysteamine | NCCS | 1313.3 | Standard polar | 33892256 | | Cysteamine | NCCS | 686.8 | Standard non polar | 33892256 | | Cysteamine | NCCS | 767.8 | Semi standard non polar | 33892256 |
Derivatized| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| Cysteamine,1TMS,isomer #1 | C[Si](C)(C)SCCN | 999.4 | Semi standard non polar | 33892256 | | Cysteamine,1TMS,isomer #1 | C[Si](C)(C)SCCN | 1000.8 | Standard non polar | 33892256 | | Cysteamine,1TMS,isomer #1 | C[Si](C)(C)SCCN | 1500.5 | Standard polar | 33892256 | | Cysteamine,1TMS,isomer #2 | C[Si](C)(C)NCCS | 1046.5 | Semi standard non polar | 33892256 | | Cysteamine,1TMS,isomer #2 | C[Si](C)(C)NCCS | 887.9 | Standard non polar | 33892256 | | Cysteamine,1TMS,isomer #2 | C[Si](C)(C)NCCS | 1266.8 | Standard polar | 33892256 | | Cysteamine,2TMS,isomer #1 | C[Si](C)(C)NCCS[Si](C)(C)C | 1203.6 | Semi standard non polar | 33892256 | | Cysteamine,2TMS,isomer #1 | C[Si](C)(C)NCCS[Si](C)(C)C | 1217.9 | Standard non polar | 33892256 | | Cysteamine,2TMS,isomer #1 | C[Si](C)(C)NCCS[Si](C)(C)C | 1253.5 | Standard polar | 33892256 | | Cysteamine,2TMS,isomer #2 | C[Si](C)(C)N(CCS)[Si](C)(C)C | 1270.3 | Semi standard non polar | 33892256 | | Cysteamine,2TMS,isomer #2 | C[Si](C)(C)N(CCS)[Si](C)(C)C | 1210.9 | Standard non polar | 33892256 | | Cysteamine,2TMS,isomer #2 | C[Si](C)(C)N(CCS)[Si](C)(C)C | 1279.0 | Standard polar | 33892256 | | Cysteamine,3TMS,isomer #1 | C[Si](C)(C)SCCN([Si](C)(C)C)[Si](C)(C)C | 1467.9 | Semi standard non polar | 33892256 | | Cysteamine,3TMS,isomer #1 | C[Si](C)(C)SCCN([Si](C)(C)C)[Si](C)(C)C | 1465.6 | Standard non polar | 33892256 | | Cysteamine,3TMS,isomer #1 | C[Si](C)(C)SCCN([Si](C)(C)C)[Si](C)(C)C | 1295.4 | Standard polar | 33892256 | | Cysteamine,1TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)SCCN | 1206.7 | Semi standard non polar | 33892256 | | Cysteamine,1TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)SCCN | 1250.7 | Standard non polar | 33892256 | | Cysteamine,1TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)SCCN | 1643.3 | Standard polar | 33892256 | | Cysteamine,1TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)NCCS | 1229.9 | Semi standard non polar | 33892256 | | Cysteamine,1TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)NCCS | 1152.8 | Standard non polar | 33892256 | | Cysteamine,1TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)NCCS | 1445.3 | Standard polar | 33892256 | | Cysteamine,2TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)NCCS[Si](C)(C)C(C)(C)C | 1671.0 | Semi standard non polar | 33892256 | | Cysteamine,2TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)NCCS[Si](C)(C)C(C)(C)C | 1624.5 | Standard non polar | 33892256 | | Cysteamine,2TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)NCCS[Si](C)(C)C(C)(C)C | 1521.9 | Standard polar | 33892256 | | Cysteamine,2TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)N(CCS)[Si](C)(C)C(C)(C)C | 1670.3 | Semi standard non polar | 33892256 | | Cysteamine,2TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)N(CCS)[Si](C)(C)C(C)(C)C | 1619.0 | Standard non polar | 33892256 | | Cysteamine,2TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)N(CCS)[Si](C)(C)C(C)(C)C | 1487.2 | Standard polar | 33892256 | | Cysteamine,3TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)SCCN([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2090.0 | Semi standard non polar | 33892256 | | Cysteamine,3TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)SCCN([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2051.6 | Standard non polar | 33892256 | | Cysteamine,3TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)SCCN([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 1687.2 | Standard polar | 33892256 |
| Show more...
|---|
| Spectra |
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental GC-MS | GC-MS Spectrum - Cysteamine GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (3 TMS) | splash10-00di-1900000000-287334efed4c27d47e62 | 2014-06-16 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Cysteamine GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (3 TMS) | splash10-00di-1900000000-e26f666cab18d523e475 | 2014-06-16 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Cysteamine GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (3 TMS) | splash10-00di-7900000000-25e27a3413a3e9bd6e86 | 2014-06-16 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Cysteamine GC-MS (3 TMS) | splash10-00dr-3900000000-5f3c0dc6b99382c852e2 | 2014-06-16 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Cysteamine GC-EI-TOF (Non-derivatized) | splash10-00di-1900000000-287334efed4c27d47e62 | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Cysteamine GC-EI-TOF (Non-derivatized) | splash10-00di-1900000000-e26f666cab18d523e475 | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Cysteamine GC-EI-TOF (Non-derivatized) | splash10-00di-7900000000-25e27a3413a3e9bd6e86 | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Cysteamine GC-MS (Non-derivatized) | splash10-00dr-3900000000-5f3c0dc6b99382c852e2 | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Cysteamine GC-MS (Non-derivatized) | splash10-00dr-3900000000-5f3c0dc6b99382c852e2 | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Cysteamine GC-EI-TOF (Non-derivatized) | splash10-00di-2900000000-ba8ae281d3d77ff202c1 | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Cysteamine GC-MS (Non-derivatized) - 70eV, Positive | splash10-003u-9000000000-48a76c3cb5971165dc16 | 2017-08-28 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Cysteamine GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental LC-MS/MS | LC-MS/MS Spectrum - Cysteamine Quattro_QQQ 10V, Positive-QTOF (Annotated) | splash10-03di-9000000000-0fdf5bdcf8a4a1bf9afb | 2012-07-25 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Cysteamine Quattro_QQQ 25V, Positive-QTOF (Annotated) | splash10-03di-9000000000-f99f2f10c6728595d6ea | 2012-07-25 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Cysteamine Quattro_QQQ 40V, Positive-QTOF (Annotated) | splash10-01t9-9000000000-fa7f96e00debe1bebf70 | 2012-07-25 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Cysteamine 35V, Positive-QTOF | splash10-0udi-9000000000-f7ecadf4e8c1d670f5a7 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Cysteamine 10V, Positive-QTOF | splash10-004i-9000000000-9bcb6f0736d473b67041 | 2017-07-26 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Cysteamine 20V, Positive-QTOF | splash10-01t9-9000000000-2607b3d0e4b061c850bd | 2017-07-26 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Cysteamine 40V, Positive-QTOF | splash10-03di-9000000000-1455192f11f88cd089c5 | 2017-07-26 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Cysteamine 10V, Negative-QTOF | splash10-004i-9000000000-e7f199c21a894c5cf75c | 2017-07-26 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Cysteamine 20V, Negative-QTOF | splash10-004l-9000000000-148162e343b3a9f7373b | 2017-07-26 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Cysteamine 40V, Negative-QTOF | splash10-001l-9000000000-205aabf68c202f080d99 | 2017-07-26 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Cysteamine 10V, Negative-QTOF | splash10-004i-9000000000-b167802490bdc502828d | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Cysteamine 20V, Negative-QTOF | splash10-00b9-9000000000-006d44449441398ce13b | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Cysteamine 40V, Negative-QTOF | splash10-057i-9000000000-b2c213fcf6e17049140b | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Cysteamine 10V, Positive-QTOF | splash10-03di-9000000000-ff9c437be9955d96c16e | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Cysteamine 20V, Positive-QTOF | splash10-03di-9000000000-ff9c437be9955d96c16e | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Cysteamine 40V, Positive-QTOF | splash10-03di-9000000000-947d309fccf2ffba996f | 2021-09-22 | Wishart Lab | View Spectrum |
NMR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Experimental 1D NMR | 1H NMR Spectrum (1D, 500 MHz, H2O, experimental) | 2012-12-05 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Experimental 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 500 MHz, H2O, experimental) | 2012-12-05 | Wishart Lab | View Spectrum |
IR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M-H]-) | 2023-02-03 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M+H]+) | 2023-02-03 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M+Na]+) | 2023-02-03 | FELIX lab | View Spectrum |
| Show more...
|---|
| Biological Properties |
|---|
| Cellular Locations | - Cytoplasm (predicted from logP)
|
|---|
| Biospecimen Locations | |
|---|
| Tissue Locations | - Leukocyte
- Pancreas
- Skeletal Muscle
- Spleen
|
|---|
| Pathways | |
|---|
| Normal Concentrations |
|---|
| |
| Blood | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Urine | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Urine | Detected and Quantified | <1 nmol/mmol creatinine | Adult (>18 years old) | Both | Normal | | details |
|
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| Associated Disorders and Diseases |
|---|
| Disease References | None |
|---|
| Associated OMIM IDs | None |
|---|
| External Links |
|---|
| DrugBank ID | DB00847 |
|---|
| Phenol Explorer Compound ID | Not Available |
|---|
| FooDB ID | FDB023091 |
|---|
| KNApSAcK ID | Not Available |
|---|
| Chemspider ID | 5834 |
|---|
| KEGG Compound ID | C01678 |
|---|
| BioCyc ID | CPD-239 |
|---|
| BiGG ID | 1808054 |
|---|
| Wikipedia Link | Cysteamine |
|---|
| METLIN ID | 3222 |
|---|
| PubChem Compound | 6058 |
|---|
| PDB ID | Not Available |
|---|
| ChEBI ID | 17141 |
|---|
| Food Biomarker Ontology | Not Available |
|---|
| VMH ID | CYSAM |
|---|
| MarkerDB ID | Not Available |
|---|
| Good Scents ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| General References | - Dominy JE Jr, Simmons CR, Hirschberger LL, Hwang J, Coloso RM, Stipanuk MH: Discovery and characterization of a second mammalian thiol dioxygenase, cysteamine dioxygenase. J Biol Chem. 2007 Aug 31;282(35):25189-98. Epub 2007 Jun 20. [PubMed:17581819 ]
- Pastore A, Massoud R, Motti C, Lo Russo A, Fucci G, Cortese C, Federici G: Fully automated assay for total homocysteine, cysteine, cysteinylglycine, glutathione, cysteamine, and 2-mercaptopropionylglycine in plasma and urine. Clin Chem. 1998 Apr;44(4):825-32. [PubMed:9554495 ]
- Sonies BC, Almajid P, Kleta R, Bernardini I, Gahl WA: Swallowing dysfunction in 101 patients with nephropathic cystinosis: benefit of long-term cysteamine therapy. Medicine (Baltimore). 2005 May;84(3):137-46. [PubMed:15879904 ]
- Lochman P, Adam T, Friedecky D, Hlidkova E, Skopkova Z: High-throughput capillary electrophoretic method for determination of total aminothiols in plasma and urine. Electrophoresis. 2003 Apr;24(7-8):1200-7. [PubMed:12707912 ]
- Chakrabarti MC, Le N, Paik CH, De Graff WG, Carrasquillo JA: Prevention of radiolysis of monoclonal antibody during labeling. J Nucl Med. 1996 Aug;37(8):1384-8. [PubMed:8708780 ]
- Skov Olsen P: Role of epidermal growth factor in gastroduodenal mucosal protection. J Clin Gastroenterol. 1988;10 Suppl 1:S146-51. [PubMed:3053882 ]
- Gahl WA, Schneider JA, Schulman JD, Thoene JG, Reed GF: Predicted reciprocal serum creatinine at age 10 years as a measure of renal function in children with nephropathic cystinosis treated with oral cysteamine. Pediatr Nephrol. 1990 Mar;4(2):129-35. [PubMed:2397178 ]
- Smolin LA, Clark KF, Thoene JG, Gahl WA, Schneider JA: A comparison of the effectiveness of cysteamine and phosphocysteamine in elevating plasma cysteamine concentration and decreasing leukocyte free cystine in nephropathic cystinosis. Pediatr Res. 1988 Jun;23(6):616-20. [PubMed:3393396 ]
- Levtchenko EN, van Dael CM, de Graaf-Hess AC, Wilmer MJ, van den Heuvel LP, Monnens LA, Blom HJ: Strict cysteamine dose regimen is required to prevent nocturnal cystine accumulation in cystinosis. Pediatr Nephrol. 2006 Jan;21(1):110-3. Epub 2005 Oct 27. [PubMed:16252107 ]
- Liu YC, Wang CM, Hsiung KP: Comparison of different protein immobilization methods on quartz crystal microbalance surface in flow injection immunoassay. Anal Biochem. 2001 Dec 15;299(2):130-5. [PubMed:11730334 ]
- Yudkoff M, Foreman JW, Segal S: Effects of cysteamine therapy in nephropathic cystinosis. N Engl J Med. 1981 Jan 15;304(3):141-5. [PubMed:7442733 ]
- Gahl WA, Charnas L, Markello TC, Bernardini I, Ishak KG, Dalakas MC: Parenchymal organ cystine depletion with long-term cysteamine therapy. Biochem Med Metab Biol. 1992 Dec;48(3):275-85. [PubMed:1476793 ]
- Geelen JM, Monnens LA, Levtchenko EN: Follow-up and treatment of adults with cystinosis in the Netherlands. Nephrol Dial Transplant. 2002 Oct;17(10):1766-70. [PubMed:12270982 ]
- Stachowicz M, Lehmann B, Tibi A, Prognon P, Daurat V, Pradeau D: Determination of total cysteamine in human serum by a high-performance liquid chromatography with fluorescence detection. J Pharm Biomed Anal. 1998 Aug;17(4-5):767-73. [PubMed:9682161 ]
- Orloff S, Butler JD, Towne D, Mukherjee AB, Schulman JD: Pantetheinase activity and cysteamine content in cystinotic and normal fibroblasts and leukocytes. Pediatr Res. 1981 Jul;15(7):1063-7. [PubMed:7254953 ]
- de Graaf-Hess A, Trijbels F, Blom H: New method for determining cystine in leukocytes and fibroblasts. Clin Chem. 1999 Dec;45(12):2224-8. [PubMed:10585356 ]
- Chmiel J, Kopczynski Z, Rybczynska M: The influence of the known radioprotective compounds on the metabolism of red blood cells. I. Effect of cysteamine on the cellular level of the intermediates and coenzymes. Pol J Pharmacol Pharm. 1976;28(2):113-21. [PubMed:934944 ]
- Schneider JA, Clark KF, Greene AA, Reisch JS, Markello TC, Gahl WA, Thoene JG, Noonan PK, Berry KA: Recent advances in the treatment of cystinosis. J Inherit Metab Dis. 1995;18(4):387-97. [PubMed:7494398 ]
- Thiele I, Swainston N, Fleming RM, Hoppe A, Sahoo S, Aurich MK, Haraldsdottir H, Mo ML, Rolfsson O, Stobbe MD, Thorleifsson SG, Agren R, Bolling C, Bordel S, Chavali AK, Dobson P, Dunn WB, Endler L, Hala D, Hucka M, Hull D, Jameson D, Jamshidi N, Jonsson JJ, Juty N, Keating S, Nookaew I, Le Novere N, Malys N, Mazein A, Papin JA, Price ND, Selkov E Sr, Sigurdsson MI, Simeonidis E, Sonnenschein N, Smallbone K, Sorokin A, van Beek JH, Weichart D, Goryanin I, Nielsen J, Westerhoff HV, Kell DB, Mendes P, Palsson BO: A community-driven global reconstruction of human metabolism. Nat Biotechnol. 2013 May;31(5):419-25. doi: 10.1038/nbt.2488. Epub 2013 Mar 3. [PubMed:23455439 ]
| Show more...
|---|