| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Detected and Quantified |
|---|
| Creation Date | 2006-08-13 02:31:31 UTC |
|---|
| Update Date | 2022-03-07 02:49:19 UTC |
|---|
| HMDB ID | HMDB0003648 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Retinyl palmitate |
|---|
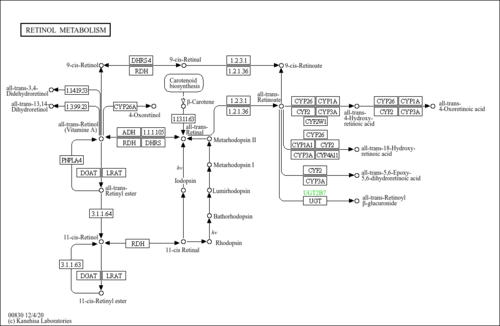
| Description | Retinyl palmitate, or vitamin A palmitate, is a common vitamin supplement, with formula C36H60O2. It is available in both oral and injectable forms for treatment of vitamin A deficiency, under the brand names Aquasol and Palmitate. Retinyl palmitate is an alternate for retinyl acetate in vitamin A supplements, and is available in oily or dry forms. It is a pre-formed version of vitamin A, and can thus be realistically over-dosed, unlike beta-carotene. |
|---|
| Structure | CCCCCCCCCCCCCCCC(=O)OC\C=C(/C)\C=C\C=C(/C)\C=C\C1=C(C)CCCC1(C)C InChI=1S/C36H60O2/c1-7-8-9-10-11-12-13-14-15-16-17-18-19-25-35(37)38-30-28-32(3)23-20-22-31(2)26-27-34-33(4)24-21-29-36(34,5)6/h20,22-23,26-28H,7-19,21,24-25,29-30H2,1-6H3/b23-20+,27-26+,31-22+,32-28+ |
|---|
| Synonyms | | Value | Source |
|---|
| (2E,4E,6E,8E)-3,7-Dimethyl-9-(2,6,6-trimethyl-1-cyclohexenyl)nona-2,4,6,8-tetraenyl hexadecanoate | ChEBI | | (2E,4E,6E,8E)-3,7-Dimethyl-9-(2,6,6-trimethyl-cyclohex-1-enyl)-nona-2,4,6,8,tetraenyl hexadecanoic acid ester | ChEBI | | Afaxin | ChEBI | | all-trans-Retinol palmitate | ChEBI | | all-trans-Retinyl hexadecanoate | ChEBI | | Alphalin | ChEBI | | Aquasol a | ChEBI | | Arovit | ChEBI | | Chocola a | ChEBI | | O(15)-Hexadecanoylretinol | ChEBI | | Optovit-a | ChEBI | | Retinol hexadecanoate | ChEBI | | Retinol palmitate | ChEBI | | Retinyl hexadecanoate | ChEBI | | trans-Retinol palmitate | ChEBI | | trans-Retinyl palmitate | ChEBI | | Vitamin a palmitate | ChEBI | | all-trans-Retinyl palmitate | Kegg | | (2E,4E,6E,8E)-3,7-Dimethyl-9-(2,6,6-trimethyl-1-cyclohexenyl)nona-2,4,6,8-tetraenyl hexadecanoic acid | Generator | | (2E,4E,6E,8E)-3,7-Dimethyl-9-(2,6,6-trimethyl-cyclohex-1-enyl)-nona-2,4,6,8,tetraenyl hexadecanoate ester | Generator | | all-trans-Retinol palmitic acid | Generator | | all-trans-Retinyl hexadecanoic acid | Generator | | Retinol hexadecanoic acid | Generator | | Retinol palmitic acid | Generator | | Retinyl hexadecanoic acid | Generator | | trans-Retinol palmitic acid | Generator | | trans-Retinyl palmitic acid | Generator | | Vitamin a palmitic acid | Generator | | all-trans-Retinyl palmitic acid | Generator | | Retinyl palmitic acid | Generator | | all-trans-Vitamin a palmitate | HMDB | | Aquapalm | HMDB | | Axerophthol palmitate | HMDB | | Dispatabs tabs | HMDB | | Ester found in fish liver oils | HMDB | | Lutavit a 500 plus | HMDB | | Myvak | HMDB | | Myvax | HMDB | | Optovit a | HMDB | | Testavol S | HMDB | | Vitazyme a | HMDB |
|
|---|
| Chemical Formula | C36H60O2 |
|---|
| Average Molecular Weight | 524.8604 |
|---|
| Monoisotopic Molecular Weight | 524.459331164 |
|---|
| IUPAC Name | (2E,4E,6E,8E)-3,7-dimethyl-9-(2,6,6-trimethylcyclohex-1-en-1-yl)nona-2,4,6,8-tetraen-1-yl hexadecanoate |
|---|
| Traditional Name | vitamin a palmitate |
|---|
| CAS Registry Number | 79-81-2 |
|---|
| SMILES | CCCCCCCCCCCCCCCC(=O)OC\C=C(/C)\C=C\C=C(/C)\C=C\C1=C(C)CCCC1(C)C |
|---|
| InChI Identifier | InChI=1S/C36H60O2/c1-7-8-9-10-11-12-13-14-15-16-17-18-19-25-35(37)38-30-28-32(3)23-20-22-31(2)26-27-34-33(4)24-21-29-36(34,5)6/h20,22-23,26-28H,7-19,21,24-25,29-30H2,1-6H3/b23-20+,27-26+,31-22+,32-28+ |
|---|
| InChI Key | VYGQUTWHTHXGQB-FFHKNEKCSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as wax monoesters. These are waxes bearing an ester group at exactly one position. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty acid esters |
|---|
| Direct Parent | Wax monoesters |
|---|
| Alternative Parents | |
|---|
| Substituents | - Wax monoester skeleton
- Retinoid skeleton
- Diterpenoid
- Fatty alcohol ester
- Carboxylic acid ester
- Monocarboxylic acid or derivatives
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic homomonocyclic compound
|
|---|
| Molecular Framework | Aliphatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Physiological effect | Not Available |
|---|
| Disposition | |
|---|
| Process | |
|---|
| Role | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | |
|---|
| Experimental Chromatographic Properties | Not Available |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Retention Times Underivatized| Chromatographic Method | Retention Time | Reference |
|---|
| Measured using a Waters Acquity ultraperformance liquid chromatography (UPLC) ethylene-bridged hybrid (BEH) C18 column (100 mm × 2.1 mm; 1.7 μmparticle diameter)). Predicted by Afia on May 17, 2022. | 7.97 minutes | 32390414 | | Predicted by Siyang on May 30, 2022 | 45.609 minutes | 33406817 | | Predicted by Siyang using ReTip algorithm on June 8, 2022 | 0.96 minutes | 32390414 | | Fem_Long = Waters ACQUITY UPLC HSS T3 C18 with Water:MeOH and 0.1% Formic Acid | 5494.9 seconds | 40023050 | | Fem_Lipids = Ascentis Express C18 with (60:40 water:ACN):(90:10 IPA:ACN) and 10mM NH4COOH + 0.1% Formic Acid | 1410.3 seconds | 40023050 | | Life_Old = Waters ACQUITY UPLC BEH C18 with Water:(20:80 acetone:ACN) and 0.1% Formic Acid | 523.3 seconds | 40023050 | | Life_New = RP Waters ACQUITY UPLC HSS T3 C18 with Water:(30:70 MeOH:ACN) and 0.1% Formic Acid | 811.1 seconds | 40023050 | | RIKEN = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 994.8 seconds | 40023050 | | Eawag_XBridgeC18 = XBridge C18 3.5u 2.1x50 mm with Water:MeOH and 0.1% Formic Acid | 1956.7 seconds | 40023050 | | BfG_NTS_RP1 =Agilent Zorbax Eclipse Plus C18 (2.1 mm x 150 mm, 3.5 um) with Water:ACN and 0.1% Formic Acid | 1628.0 seconds | 40023050 | | HILIC_BDD_2 = Merck SeQuant ZIC-HILIC with ACN(0.1% formic acid):water(16 mM ammonium formate) | 124.7 seconds | 40023050 | | UniToyama_Atlantis = RP Waters Atlantis T3 (2.1 x 150 mm, 5 um) with ACN:Water and 0.1% Formic Acid | 4053.9 seconds | 40023050 | | BDD_C18 = Hypersil Gold 1.9µm C18 with Water:ACN and 0.1% Formic Acid | 1233.5 seconds | 40023050 | | UFZ_Phenomenex = Kinetex Core-Shell C18 2.6 um, 3.0 x 100 mm, Phenomenex with Water:MeOH and 0.1% Formic Acid | 3044.3 seconds | 40023050 | | SNU_RIKEN_POS = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 1591.0 seconds | 40023050 | | RPMMFDA = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 1013.1 seconds | 40023050 | | MTBLS87 = Merck SeQuant ZIC-pHILIC column with ACN:Water and :ammonium carbonate | 953.9 seconds | 40023050 | | KI_GIAR_zic_HILIC_pH2_7 = Merck SeQuant ZIC-HILIC with ACN:Water and 0.1% FA | 1170.3 seconds | 40023050 | | Meister zic-pHILIC pH9.3 = Merck SeQuant ZIC-pHILIC column with ACN:Water 5mM NH4Ac pH9.3 and 5mM ammonium acetate in water | 10.5 seconds | 40023050 |
Predicted Kovats Retention IndicesUnderivatized |
|---|
| General References | - Gylling H, Relas H, Miettinen HE, Radhakrishnan R, Miettinen TA: Delayed postprandial retinyl palmitate and squalene removal in a patient heterozygous for apolipoprotein A-IFIN mutation (Leu 159-->Arg) and low HDL cholesterol level without coronary artery disease. Atherosclerosis. 1996 Dec 20;127(2):239-43. [PubMed:9125314 ]
- Relas H, Gylling H, Miettinen TA: Effect of stanol ester on postabsorptive squalene and retinyl palmitate. Metabolism. 2000 Apr;49(4):473-8. [PubMed:10778871 ]
- Antille C, Tran C, Sorg O, Carraux P, Didierjean L, Saurat JH: Vitamin A exerts a photoprotective action in skin by absorbing ultraviolet B radiation. J Invest Dermatol. 2003 Nov;121(5):1163-7. [PubMed:14708621 ]
- Epler KS, Ziegler RG, Craft NE: Liquid chromatographic method for the determination of carotenoids, retinoids and tocopherols in human serum and in food. J Chromatogr. 1993 Sep 8;619(1):37-48. [PubMed:8245162 ]
- Ribaya-Mercado JD, Blanco MC, Fox JG, Russell RM: High concentrations of vitamin A esters circulate primarily as retinyl stearate and are stored primarily as retinyl palmitate in ferret tissues. J Am Coll Nutr. 1994 Feb;13(1):83-6. [PubMed:8157860 ]
- Thomas JB, Kline MC, Schiller SB, Ellerbe PM, Sniegoski LT, Duewer DL, Sharpless KE: Certification of fat-soluble vitamins, carotenoids, and cholesterol in human serum: Standard Reference Material 968b. Anal Bioanal Chem. 1996 Aug;356(1):1-9. [PubMed:15045249 ]
- Fernandez-Miranda C, Cancelas P, Sanz M, Porres A, Gamez Gerique J: Influence of apolipoprotein-E phenotypes on postprandial lipoprotein metabolism after three different fat loads. Nutrition. 2001 Jul-Aug;17(7-8):529-33. [PubMed:11448569 ]
- Weintraub M, Grosskopf I, Trostanesky Y, Charach G, Rubinstein A, Stern N: Thyroxine replacement therapy enhances clearance of chylomicron remnants in patients with hypothyroidism. J Clin Endocrinol Metab. 1999 Jul;84(7):2532-6. [PubMed:10404832 ]
- Lindstrom MB, Sternby B, Borgstrom B: Concerted action of human carboxyl ester lipase and pancreatic lipase during lipid digestion in vitro: importance of the physicochemical state of the substrate. Biochim Biophys Acta. 1988 Mar 25;959(2):178-84. [PubMed:3349096 ]
- Gimeno A, Zaragoza R, Vivo-Sese I, Vina JR, Miralles VJ: Retinol, at concentrations greater than the physiological limit, induces oxidative stress and apoptosis in human dermal fibroblasts. Exp Dermatol. 2004 Jan;13(1):45-54. [PubMed:15009115 ]
- Kobayashi TK, Tsubota K, Takamura E, Sawa M, Ohashi Y, Usui M: Effect of retinol palmitate as a treatment for dry eye: a cytological evaluation. Ophthalmologica. 1997;211(6):358-61. [PubMed:9380354 ]
- Foger B, Drexel H, Hopferwieser T, Miesenbock G, Ritsch A, Lechleitner M, Trobinger G, Patsch JR: Fenofibrate improves postprandial chylomicron clearance in II B hyperlipoproteinemia. Clin Investig. 1994 Mar;72(4):294-301. [PubMed:8043977 ]
- Van Lieshout M, West CE, Van De Bovenkamp P, Wang Y, Sun Y, Van Breemen RB, Muhilal DP, Verhoeven MA, Creemers AF, Lugtenburg J: Extraction of carotenoids from feces, enabling the bioavailability of beta-carotene to be studied in Indonesian children. J Agric Food Chem. 2003 Aug 13;51(17):5123-30. [PubMed:12903979 ]
- Zampelas A, Ah-Sing E, Chakraboraty J, Murphy M, Peel A, Wright J, Williams CM: The use of retinyl palmitate to measure clearance of chylomicrons and chylomicron remnants following meals of different fatty acid compositions. Biochem Soc Trans. 1993 May;21(2):137S. [PubMed:8359391 ]
- Hartmann S, Froescheis O, Ringenbach F, Wyss R, Bucheli F, Bischof S, Bausch J, Wiegand UW: Determination of retinol and retinyl esters in human plasma by high-performance liquid chromatography with automated column switching and ultraviolet detection. J Chromatogr B Biomed Sci Appl. 2001 Feb 25;751(2):265-75. [PubMed:11236082 ]
- Tzimas G, Collins MD, Burgin H, Hummler H, Nau H: Embryotoxic doses of vitamin A to rabbits result in low plasma but high embryonic concentrations of all-trans-retinoic acid: risk of vitamin A exposure in humans. J Nutr. 1996 Sep;126(9):2159-71. [PubMed:8814204 ]
- Guerci B, Paul JL, Hadjadj S, Durlach V, Verges B, Attia N, Girard-Globa A, Drouin P: Analysis of the postprandial lipid metabolism: use of a 3-point test. Diabetes Metab. 2001 Sep;27(4 Pt 1):449-57. [PubMed:11547218 ]
- de Bruin TW, Brouwer CB, van Linde-Sibenius Trip M, Jansen H, Erkelens DW: Different postprandial metabolism of olive oil and soybean oil: a possible mechanism of the high-density lipoprotein conserving effect of olive oil. Am J Clin Nutr. 1993 Oct;58(4):477-83. [PubMed:8379502 ]
- Martins IJ, Hopkins L, Joll CA, Redgrave TG: Interactions between model triacylglycerol-rich lipoproteins and high-density lipoproteins in rat, rabbit and man. Biochim Biophys Acta. 1991 Feb 5;1081(3):328-38. [PubMed:1998751 ]
- Schindler R, Klopp A: Transport of esterified retinol in fasting human blood. Int J Vitam Nutr Res. 1986;56(1):21-7. [PubMed:3710714 ]
|
|---|