| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Detected but not Quantified |
|---|
| Creation Date | 2006-05-22 15:12:38 UTC |
|---|
| Update Date | 2020-04-22 23:33:23 UTC |
|---|
| HMDB ID | HMDB0003178 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
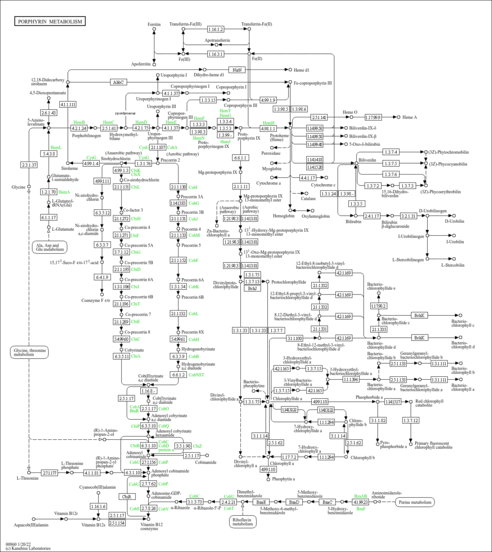
| Common Name | Heme |
|---|
| Description | Heme is the color-furnishing portion of hemoglobin. It is found free in tissues and as the prosthetic group in many hemeproteins. A heme or haem is a prosthetic group that consists of an iron atom contained in the center of a large heterocyclic organic ring called a porphyrin. Not all porphyrins contain iron, but a substantial fraction of porphyrin-containing metalloproteins have heme as their prosthetic subunit; these are known as hemoproteins. |
|---|
| Structure | CC1=C(CCC(O)=O)C2=CC3=[N+]4C(=CC5=C(C)C(C=C)=C6C=C7C(C)=C(C=C)C8=[N+]7[Fe--]4(N2C1=C8)N56)C(C)=C3CCC(O)=O InChI=1S/C34H34N4O4.Fe/c1-7-21-17(3)25-13-26-19(5)23(9-11-33(39)40)31(37-26)16-32-24(10-12-34(41)42)20(6)28(38-32)15-30-22(8-2)18(4)27(36-30)14-29(21)35-25;/h7-8,13-16H,1-2,9-12H2,3-6H3,(H4,35,36,37,38,39,40,41,42);/q;+2/p-2/b25-13-,26-13-,27-14-,28-15-,29-14-,30-15-,31-16-,32-16-; |
|---|
| Synonyms | | Value | Source |
|---|
| [3,7,12,17-Tetramethyl-8,13-divinylporphyrin-2,18-dipropanoato(2-)]iron(II) | ChEBI | | [Fe(ppix)] | ChEBI | | Fe(ppix) | ChEBI | | Ferroprotoheme | ChEBI | | Ferroprotoporphyrin IX | ChEBI | | Ferrous protoheme | ChEBI | | Ferrous protoheme IX | ChEBI | | Haem | ChEBI | | Iron(II) protoporphyrin IX | ChEBI | | Protoferroheme | ChEBI | | Protoheme | ChEBI | | Heme b | Kegg | | Protoheme IX | Kegg | | Heme | ChEBI |
|
|---|
| Chemical Formula | C34H32FeN4O4 |
|---|
| Average Molecular Weight | 616.487 |
|---|
| Monoisotopic Molecular Weight | 616.177297665 |
|---|
| IUPAC Name | 4,20-bis(2-carboxyethyl)-10,15-diethenyl-5,9,14,19-tetramethyl-2lambda5,22,23lambda5,25-tetraaza-1-ferraoctacyclo[11.9.1.1^{1,8}.1^{3,21}.0^{2,6}.0^{16,23}.0^{18,22}.0^{11,25}]pentacosa-2,4,6,8,10,12,14,16(23),17,19,21(24)-undecaene-2,23-bis(ylium)-1,1-diuide |
|---|
| Traditional Name | 4,20-bis(2-carboxyethyl)-10,15-diethenyl-5,9,14,19-tetramethyl-2lambda5,22,23lambda5,25-tetraaza-1-ferraoctacyclo[11.9.1.1^{1,8}.1^{3,21}.0^{2,6}.0^{16,23}.0^{18,22}.0^{11,25}]pentacosa-2,4,6,8,10,12,14,16(23),17,19,21(24)-undecaene-2,23-bis(ylium)-1,1-diuide |
|---|
| CAS Registry Number | 14875-96-8 |
|---|
| SMILES | CC1=C(CCC(O)=O)C2=CC3=[N+]4C(=CC5=C(C)C(C=C)=C6C=C7C(C)=C(C=C)C8=[N+]7[Fe--]4(N2C1=C8)N56)C(C)=C3CCC(O)=O |
|---|
| InChI Identifier | InChI=1S/C34H34N4O4.Fe/c1-7-21-17(3)25-13-26-19(5)23(9-11-33(39)40)31(37-26)16-32-24(10-12-34(41)42)20(6)28(38-32)15-30-22(8-2)18(4)27(36-30)14-29(21)35-25;/h7-8,13-16H,1-2,9-12H2,3-6H3,(H4,35,36,37,38,39,40,41,42);/q;+2/p-2/b25-13-,26-13-,27-14-,28-15-,29-14-,30-15-,31-16-,32-16-; |
|---|
| InChI Key | KABFMIBPWCXCRK-RGGAHWMASA-L |
|---|
| Chemical Taxonomy |
|---|
| Classification | Not classified |
|---|
| Ontology |
|---|
| Not Available | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Not Available |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Retention Times Underivatized| Chromatographic Method | Retention Time | Reference |
|---|
| Fem_Long = Waters ACQUITY UPLC HSS T3 C18 with Water:MeOH and 0.1% Formic Acid | 2953.5 seconds | 40023050 | | Fem_Lipids = Ascentis Express C18 with (60:40 water:ACN):(90:10 IPA:ACN) and 10mM NH4COOH + 0.1% Formic Acid | 188.2 seconds | 40023050 | | Life_Old = Waters ACQUITY UPLC BEH C18 with Water:(20:80 acetone:ACN) and 0.1% Formic Acid | 230.0 seconds | 40023050 | | Life_New = RP Waters ACQUITY UPLC HSS T3 C18 with Water:(30:70 MeOH:ACN) and 0.1% Formic Acid | 167.6 seconds | 40023050 | | RIKEN = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 103.0 seconds | 40023050 | | Eawag_XBridgeC18 = XBridge C18 3.5u 2.1x50 mm with Water:MeOH and 0.1% Formic Acid | 586.6 seconds | 40023050 | | BfG_NTS_RP1 =Agilent Zorbax Eclipse Plus C18 (2.1 mm x 150 mm, 3.5 um) with Water:ACN and 0.1% Formic Acid | 647.3 seconds | 40023050 | | HILIC_BDD_2 = Merck SeQuant ZIC-HILIC with ACN(0.1% formic acid):water(16 mM ammonium formate) | 89.1 seconds | 40023050 | | UniToyama_Atlantis = RP Waters Atlantis T3 (2.1 x 150 mm, 5 um) with ACN:Water and 0.1% Formic Acid | 1257.5 seconds | 40023050 | | BDD_C18 = Hypersil Gold 1.9µm C18 with Water:ACN and 0.1% Formic Acid | 503.2 seconds | 40023050 | | UFZ_Phenomenex = Kinetex Core-Shell C18 2.6 um, 3.0 x 100 mm, Phenomenex with Water:MeOH and 0.1% Formic Acid | 1795.5 seconds | 40023050 | | SNU_RIKEN_POS = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 398.5 seconds | 40023050 | | RPMMFDA = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 387.5 seconds | 40023050 | | MTBLS87 = Merck SeQuant ZIC-pHILIC column with ACN:Water and :ammonium carbonate | 214.0 seconds | 40023050 | | KI_GIAR_zic_HILIC_pH2_7 = Merck SeQuant ZIC-HILIC with ACN:Water and 0.1% FA | 271.4 seconds | 40023050 | | Meister zic-pHILIC pH9.3 = Merck SeQuant ZIC-pHILIC column with ACN:Water 5mM NH4Ac pH9.3 and 5mM ammonium acetate in water | 20.7 seconds | 40023050 |
Predicted Kovats Retention IndicesUnderivatizedDerivatized| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| Heme,1TMS,isomer #1 | C=CC1=C(C)C2=CC3=C(C=C)C(C)=C4C=C5C(C)=C(CCC(=O)O)C6=[N+]5[Fe-2]5(N43)N3C(=CC1=[N+]25)C(C)=C(CCC(=O)O[Si](C)(C)C)C3=C6 | 4869.2 | Semi standard non polar | 33892256 | | Heme,1TMS,isomer #2 | C=CC1=C(C)C2=CC3=C(C=C)C(C)=C4C=C5C(C)=C(CCC(=O)O[Si](C)(C)C)C6=[N+]5[Fe-2]5(N43)N3C(=CC1=[N+]25)C(C)=C(CCC(=O)O)C3=C6 | 4882.0 | Semi standard non polar | 33892256 | | Heme,2TMS,isomer #1 | C=CC1=C(C)C2=CC3=C(C=C)C(C)=C4C=C5C(C)=C(CCC(=O)O[Si](C)(C)C)C6=[N+]5[Fe-2]5(N43)N3C(=CC1=[N+]25)C(C)=C(CCC(=O)O[Si](C)(C)C)C3=C6 | 4794.0 | Semi standard non polar | 33892256 | | Heme,1TBDMS,isomer #1 | C=CC1=C(C)C2=CC3=C(C=C)C(C)=C4C=C5C(C)=C(CCC(=O)O)C6=[N+]5[Fe-2]5(N43)N3C(=CC1=[N+]25)C(C)=C(CCC(=O)O[Si](C)(C)C(C)(C)C)C3=C6 | 5039.4 | Semi standard non polar | 33892256 | | Heme,1TBDMS,isomer #2 | C=CC1=C(C)C2=CC3=C(C=C)C(C)=C4C=C5C(C)=C(CCC(=O)O[Si](C)(C)C(C)(C)C)C6=[N+]5[Fe-2]5(N43)N3C(=CC1=[N+]25)C(C)=C(CCC(=O)O)C3=C6 | 5054.8 | Semi standard non polar | 33892256 | | Heme,2TBDMS,isomer #1 | C=CC1=C(C)C2=CC3=C(C=C)C(C)=C4C=C5C(C)=C(CCC(=O)O[Si](C)(C)C(C)(C)C)C6=[N+]5[Fe-2]5(N43)N3C(=CC1=[N+]25)C(C)=C(CCC(=O)O[Si](C)(C)C(C)(C)C)C3=C6 | 5143.4 | Semi standard non polar | 33892256 |
|
|---|
| General References | - Ono F, Sharma BK, Smith CC, Burnett JW, Aurelian L: CD34+ cells in the peripheral blood transport herpes simplex virus DNA fragments to the skin of patients with erythema multiforme (HAEM). J Invest Dermatol. 2005 Jun;124(6):1215-24. [PubMed:15955097 ]
- Allhorn M, Lundqvist K, Schmidtchen A, Akerstrom B: Heme-scavenging role of alpha1-microglobulin in chronic ulcers. J Invest Dermatol. 2003 Sep;121(3):640-6. [PubMed:12925227 ]
- Walczyk T, von Blanckenburg F: Natural iron isotope variations in human blood. Science. 2002 Mar 15;295(5562):2065-6. [PubMed:11896276 ]
- Kuhnel A, Gross U, Doss MO: Hereditary coproporphyria in Germany: clinical-biochemical studies in 53 patients. Clin Biochem. 2000 Aug;33(6):465-73. [PubMed:11074238 ]
- Zhang JP, Normark S: Induction of gene expression in Escherichia coli after pilus-mediated adherence. Science. 1996 Aug 30;273(5279):1234-6. [PubMed:8703059 ]
- Taylor TD, Litt M, Kramer P, Pandolfo M, Angelini L, Nardocci N, Davis S, Pineda M, Hattori H, Flett PJ, Cilio MR, Bertini E, Hayflick SJ: Homozygosity mapping of Hallervorden-Spatz syndrome to chromosome 20p12.3-p13. Nat Genet. 1996 Dec;14(4):479-81. [PubMed:8944032 ]
- Park KK, Park JH, Jung YJ, Chung WY: Inhibitory effects of chlorophyllin, hemin and tetrakis(4-benzoic acid)porphyrin on oxidative DNA damage and mouse skin inflammation induced by 12-O-tetradecanoylphorbol-13-acetate as a possible anti-tumor promoting mechanism. Mutat Res. 2003 Dec 9;542(1-2):89-97. [PubMed:14644357 ]
- Elshenawy S, Pinney SE, Stuart T, Doulias PT, Zura G, Parry S, Elovitz MA, Bennett MJ, Bansal A, Strauss JF 3rd, Ischiropoulos H, Simmons RA: The Metabolomic Signature of the Placenta in Spontaneous Preterm Birth. Int J Mol Sci. 2020 Feb 4;21(3). pii: ijms21031043. doi: 10.3390/ijms21031043. [PubMed:32033212 ]
|
|---|