| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Expected but not Quantified |
|---|
| Creation Date | 2006-08-13 07:26:45 UTC |
|---|
| Update Date | 2022-03-07 02:49:19 UTC |
|---|
| HMDB ID | HMDB0003932 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | (S)-3-Hydroxyhexadecanoyl-CoA |
|---|
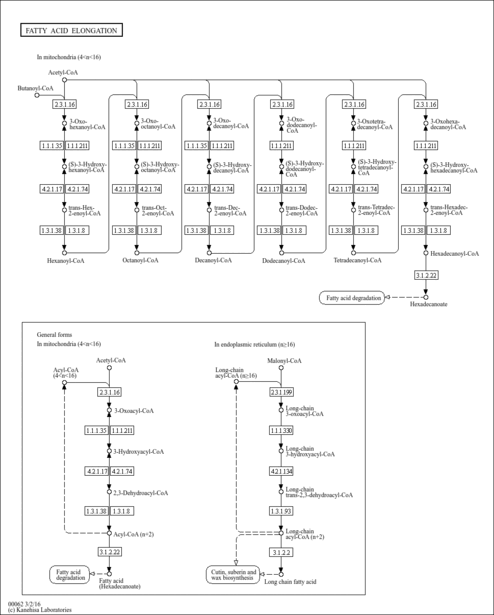
| Description | (S)-3-Hydroxyhexadecanoyl-CoA is a beta-oxidation intermediate derivative of palmitoyl-CoA and the substrate of the enzyme peroxisomal acyl-CoA thioesterase 2 (PTE-2, EC 3.1.2.2), which is localized in the peroxisome. The peroxisomal beta-oxidation system contains two sets of enzymes, one of which is involved in the oxidation of branched chain fatty acids and intermediates in the hepatic bile acid biosynthetic pathway and consists of one or two branched-chain acyl-CoA oxidase(s), a D-specific bifunctional protein and the sterol carrier-like protein x (SCPx). Peroxisomes are cellular organelles present in all eukaryotic cells. They play an indispensable role in the metabolism of a variety of lipids including very long-chain fatty acids, dicarboxylic fatty acids, bile acids, prostaglandins, leukotrienes, thromboxanes, pristanic acid, and xenobiotic fatty acids. (S)-3-Hydroxyhexadecanoyl-CoA may accumulate intracellularly in certain long-chain fatty acid/j-oxidation deficiencies. Succinate-driven synthesis of ATP from ADP and phosphate is progressively inhibited by increasing concentrations of (S)-3-Hydroxyhexadecanoyl-CoA. (PMID: 11673457 , 8739955 , 7662716 ). |
|---|
| Structure | CCCCCCCCCCCCC[C@H](O)CC(=O)SCCNC(=O)CCNC(=O)C(O)C(C)(C)COP(O)(=O)OP(O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1OP(O)(O)=O)N1C=NC2=C(N)N=CN=C12 InChI=1S/C37H66N7O18P3S/c1-4-5-6-7-8-9-10-11-12-13-14-15-25(45)20-28(47)66-19-18-39-27(46)16-17-40-35(50)32(49)37(2,3)22-59-65(56,57)62-64(54,55)58-21-26-31(61-63(51,52)53)30(48)36(60-26)44-24-43-29-33(38)41-23-42-34(29)44/h23-26,30-32,36,45,48-49H,4-22H2,1-3H3,(H,39,46)(H,40,50)(H,54,55)(H,56,57)(H2,38,41,42)(H2,51,52,53)/t25-,26+,30+,31+,32?,36+/m0/s1 |
|---|
| Synonyms | | Value | Source |
|---|
| (S)-3-Hydroxyhexadecanoyl-coenzyme A | HMDB | | (S)-3-Hydroxypalmitoyl-coenzyme A | HMDB | | b-Hydroxypalmitoyl-CoA | HMDB | | b-Hydroxypalmitoyl-coenzyme A | HMDB | | beta-Hydroxypalmitoyl-CoA | HMDB | | beta-Hydroxypalmitoyl-coenzyme A | HMDB | | DL-3-Hydroxyhexadecanoyl-S-coenzyme A | HMDB | | DL-3-Hydroxyhexadecanoyl-scoa | HMDB | | DL-3-Hydroxyhexadecanoyl-Scoenzyme A | HMDB | | S-(3-Hydroxyhexadecanoate | HMDB | | S-(3-Hydroxyhexadecanoate)coenzyme A | HMDB | | S-(3-Hydroxyhexadecanoic acid | HMDB | | S-DL-3-Hydroxyhexadecanoate | HMDB | | S-DL-3-Hydroxyhexadecanoic acid | HMDB | | 3-Hydroxyhexadecanoyl-CoA | HMDB | | 3-Hydroxyhexadecanoyl-coenzyme A | HMDB |
|
|---|
| Chemical Formula | C37H66N7O18P3S |
|---|
| Average Molecular Weight | 1021.942 |
|---|
| Monoisotopic Molecular Weight | 1021.339788569 |
|---|
| IUPAC Name | {[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-hydroxy-2-({[hydroxy({hydroxy[3-hydroxy-3-({2-[(2-{[(3S)-3-hydroxyhexadecanoyl]sulfanyl}ethyl)carbamoyl]ethyl}carbamoyl)-2,2-dimethylpropoxy]phosphoryl}oxy)phosphoryl]oxy}methyl)oxolan-3-yl]oxy}phosphonic acid |
|---|
| Traditional Name | [(2R,3S,4R,5R)-5-(6-aminopurin-9-yl)-4-hydroxy-2-[({hydroxy[hydroxy(3-hydroxy-3-({2-[(2-{[(3S)-3-hydroxyhexadecanoyl]sulfanyl}ethyl)carbamoyl]ethyl}carbamoyl)-2,2-dimethylpropoxy)phosphoryl]oxyphosphoryl}oxy)methyl]oxolan-3-yl]oxyphosphonic acid |
|---|
| CAS Registry Number | 35106-50-4 |
|---|
| SMILES | CCCCCCCCCCCCC[C@H](O)CC(=O)SCCNC(=O)CCNC(=O)C(O)C(C)(C)COP(O)(=O)OP(O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1OP(O)(O)=O)N1C=NC2=C(N)N=CN=C12 |
|---|
| InChI Identifier | InChI=1S/C37H66N7O18P3S/c1-4-5-6-7-8-9-10-11-12-13-14-15-25(45)20-28(47)66-19-18-39-27(46)16-17-40-35(50)32(49)37(2,3)22-59-65(56,57)62-64(54,55)58-21-26-31(61-63(51,52)53)30(48)36(60-26)44-24-43-29-33(38)41-23-42-34(29)44/h23-26,30-32,36,45,48-49H,4-22H2,1-3H3,(H,39,46)(H,40,50)(H,54,55)(H,56,57)(H2,38,41,42)(H2,51,52,53)/t25-,26+,30+,31+,32?,36+/m0/s1 |
|---|
| InChI Key | DEHLMTDDPWDRDR-QQOJFMBSSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as long-chain fatty acyl coas. These are acyl CoAs where the group acylated to the coenzyme A moiety is a long aliphatic chain of 13 to 21 carbon atoms. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty acyl thioesters |
|---|
| Direct Parent | Long-chain fatty acyl CoAs |
|---|
| Alternative Parents | |
|---|
| Substituents | - Coenzyme a or derivatives
- Purine ribonucleoside 3',5'-bisphosphate
- Purine ribonucleoside bisphosphate
- Purine ribonucleoside diphosphate
- Ribonucleoside 3'-phosphate
- Pentose phosphate
- Pentose-5-phosphate
- Beta amino acid or derivatives
- Glycosyl compound
- N-glycosyl compound
- 6-aminopurine
- Monosaccharide phosphate
- Organic pyrophosphate
- Pentose monosaccharide
- Imidazopyrimidine
- Purine
- Monoalkyl phosphate
- Aminopyrimidine
- Imidolactam
- N-acyl-amine
- N-substituted imidazole
- Organic phosphoric acid derivative
- Monosaccharide
- Pyrimidine
- Alkyl phosphate
- Fatty amide
- Phosphoric acid ester
- Tetrahydrofuran
- Imidazole
- Azole
- Heteroaromatic compound
- Carbothioic s-ester
- Secondary alcohol
- Thiocarboxylic acid ester
- Carboxamide group
- Secondary carboxylic acid amide
- Amino acid or derivatives
- Sulfenyl compound
- Thiocarboxylic acid or derivatives
- Organoheterocyclic compound
- Azacycle
- Oxacycle
- Carboxylic acid derivative
- Organosulfur compound
- Organic oxygen compound
- Hydrocarbon derivative
- Carbonyl group
- Organic nitrogen compound
- Primary amine
- Organopnictogen compound
- Organic oxide
- Organooxygen compound
- Organonitrogen compound
- Alcohol
- Amine
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Physiological effect | Not Available |
|---|
| Disposition | |
|---|
| Process | |
|---|
| Role | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | 1.481 | Not Available |
|
|---|
| Experimental Chromatographic Properties | Not Available |
|---|
| Predicted Molecular Properties | | Show more...
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Kovats Retention IndicesNot Available | Show more...
|---|
| Spectra |
|---|
| MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - (S)-3-Hydroxyhexadecanoyl-CoA 10V, Positive-QTOF | splash10-000i-4903110201-5c625add946cedeba8fd | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - (S)-3-Hydroxyhexadecanoyl-CoA 20V, Positive-QTOF | splash10-000i-0913130000-d6cef11122d96c9fb376 | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - (S)-3-Hydroxyhexadecanoyl-CoA 40V, Positive-QTOF | splash10-000i-1900000100-8d7d4b3ad4d074a7843f | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - (S)-3-Hydroxyhexadecanoyl-CoA 10V, Negative-QTOF | splash10-0g1i-9670331410-7d32679c05799895d716 | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - (S)-3-Hydroxyhexadecanoyl-CoA 20V, Negative-QTOF | splash10-001i-4920201000-cd5080910a861d80fc42 | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - (S)-3-Hydroxyhexadecanoyl-CoA 40V, Negative-QTOF | splash10-057i-6900100000-fbfb99f57376aea60156 | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - (S)-3-Hydroxyhexadecanoyl-CoA 10V, Positive-QTOF | splash10-0fk9-9000000001-05072eea992abb5f2395 | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - (S)-3-Hydroxyhexadecanoyl-CoA 20V, Positive-QTOF | splash10-01yc-3000100098-6c40c412026538d46263 | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - (S)-3-Hydroxyhexadecanoyl-CoA 40V, Positive-QTOF | splash10-014i-0101490000-0ed7def0065ebb0477bb | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - (S)-3-Hydroxyhexadecanoyl-CoA 10V, Negative-QTOF | splash10-00di-9000000000-30c47ac3be95c4e52760 | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - (S)-3-Hydroxyhexadecanoyl-CoA 20V, Negative-QTOF | splash10-0l90-9200101220-df47e7774864d969c394 | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - (S)-3-Hydroxyhexadecanoyl-CoA 40V, Negative-QTOF | splash10-0fbm-9305604617-7125cce3b8fd55475b9c | 2021-09-25 | Wishart Lab | View Spectrum |
NMR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum |
| Show more...
|---|
| Biological Properties |
|---|
| Cellular Locations | |
|---|
| Biospecimen Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| Associated Disorders and Diseases |
|---|
| Disease References | None |
|---|
| Associated OMIM IDs | None |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| Phenol Explorer Compound ID | Not Available |
|---|
| FooDB ID | FDB023251 |
|---|
| KNApSAcK ID | Not Available |
|---|
| Chemspider ID | 389498 |
|---|
| KEGG Compound ID | C05258 |
|---|
| BioCyc ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PubChem Compound | 440600 |
|---|
| PDB ID | Not Available |
|---|
| ChEBI ID | 27402 |
|---|
| Food Biomarker Ontology | Not Available |
|---|
| VMH ID | 3HHDCOA |
|---|
| MarkerDB ID | Not Available |
|---|
| Good Scents ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Al-Arif, Adhid; Blecher, Melvin. Chemical synthesis of carnitine and coenzyme A esters of the b-substituted intermediates of hexadecanoic acid metabolism. Biochimica et Biophysica Acta, Lipids and Lipid Metabolism (1971), 248(3), 416-29. |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| General References | - Hunt MC, Solaas K, Kase BF, Alexson SE: Characterization of an acyl-coA thioesterase that functions as a major regulator of peroxisomal lipid metabolism. J Biol Chem. 2002 Jan 11;277(2):1128-38. Epub 2001 Oct 22. [PubMed:11673457 ]
- Ventura FV, Ruiter JP, Ijlst L, de Almeida IT, Wanders RJ: Inhibitory effect of 3-hydroxyacyl-CoAs and other long-chain fatty acid beta-oxidation intermediates on mitochondrial oxidative phosphorylation. J Inherit Metab Dis. 1996;19(2):161-4. [PubMed:8739955 ]
- Ventura FV, Ruiter JP, Ijlst L, Almeida IT, Wanders RJ: Inhibition of oxidative phosphorylation by palmitoyl-CoA in digitonin permeabilized fibroblasts: implications for long-chain fatty acid beta-oxidation disorders. Biochim Biophys Acta. 1995 Aug 15;1272(1):14-20. [PubMed:7662716 ]
|
|---|