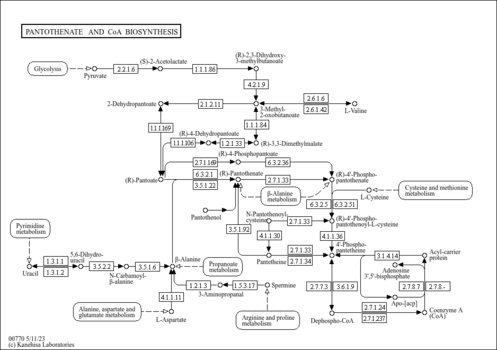
| Pantetheine,1TMS,isomer #1 | CC(C)(CO[Si](C)(C)C)C(O)C(=O)NCCC(=O)NCCS | 2527.7 | Semi standard non polar | 33892256 |
| Pantetheine,1TMS,isomer #2 | CC(C)(CO)C(O[Si](C)(C)C)C(=O)NCCC(=O)NCCS | 2470.6 | Semi standard non polar | 33892256 |
| Pantetheine,1TMS,isomer #3 | CC(C)(CO)C(O)C(=O)NCCC(=O)NCCS[Si](C)(C)C | 2579.2 | Semi standard non polar | 33892256 |
| Pantetheine,1TMS,isomer #4 | CC(C)(CO)C(O)C(=O)N(CCC(=O)NCCS)[Si](C)(C)C | 2460.5 | Semi standard non polar | 33892256 |
| Pantetheine,1TMS,isomer #5 | CC(C)(CO)C(O)C(=O)NCCC(=O)N(CCS)[Si](C)(C)C | 2473.3 | Semi standard non polar | 33892256 |
| Pantetheine,2TMS,isomer #1 | CC(C)(CO[Si](C)(C)C)C(O[Si](C)(C)C)C(=O)NCCC(=O)NCCS | 2515.4 | Semi standard non polar | 33892256 |
| Pantetheine,2TMS,isomer #10 | CC(C)(CO)C(O)C(=O)N(CCC(=O)N(CCS)[Si](C)(C)C)[Si](C)(C)C | 2440.5 | Semi standard non polar | 33892256 |
| Pantetheine,2TMS,isomer #2 | CC(C)(CO[Si](C)(C)C)C(O)C(=O)NCCC(=O)NCCS[Si](C)(C)C | 2621.4 | Semi standard non polar | 33892256 |
| Pantetheine,2TMS,isomer #3 | CC(C)(CO[Si](C)(C)C)C(O)C(=O)N(CCC(=O)NCCS)[Si](C)(C)C | 2495.9 | Semi standard non polar | 33892256 |
| Pantetheine,2TMS,isomer #4 | CC(C)(CO[Si](C)(C)C)C(O)C(=O)NCCC(=O)N(CCS)[Si](C)(C)C | 2494.8 | Semi standard non polar | 33892256 |
| Pantetheine,2TMS,isomer #5 | CC(C)(CO)C(O[Si](C)(C)C)C(=O)NCCC(=O)NCCS[Si](C)(C)C | 2542.5 | Semi standard non polar | 33892256 |
| Pantetheine,2TMS,isomer #6 | CC(C)(CO)C(O[Si](C)(C)C)C(=O)N(CCC(=O)NCCS)[Si](C)(C)C | 2472.0 | Semi standard non polar | 33892256 |
| Pantetheine,2TMS,isomer #7 | CC(C)(CO)C(O[Si](C)(C)C)C(=O)NCCC(=O)N(CCS)[Si](C)(C)C | 2432.6 | Semi standard non polar | 33892256 |
| Pantetheine,2TMS,isomer #8 | CC(C)(CO)C(O)C(=O)N(CCC(=O)NCCS[Si](C)(C)C)[Si](C)(C)C | 2561.4 | Semi standard non polar | 33892256 |
| Pantetheine,2TMS,isomer #9 | CC(C)(CO)C(O)C(=O)NCCC(=O)N(CCS[Si](C)(C)C)[Si](C)(C)C | 2615.1 | Semi standard non polar | 33892256 |
| Pantetheine,3TMS,isomer #1 | CC(C)(CO[Si](C)(C)C)C(O[Si](C)(C)C)C(=O)NCCC(=O)NCCS[Si](C)(C)C | 2574.1 | Semi standard non polar | 33892256 |
| Pantetheine,3TMS,isomer #1 | CC(C)(CO[Si](C)(C)C)C(O[Si](C)(C)C)C(=O)NCCC(=O)NCCS[Si](C)(C)C | 2503.0 | Standard non polar | 33892256 |
| Pantetheine,3TMS,isomer #1 | CC(C)(CO[Si](C)(C)C)C(O[Si](C)(C)C)C(=O)NCCC(=O)NCCS[Si](C)(C)C | 2820.4 | Standard polar | 33892256 |
| Pantetheine,3TMS,isomer #10 | CC(C)(CO)C(O)C(=O)N(CCC(=O)N(CCS[Si](C)(C)C)[Si](C)(C)C)[Si](C)(C)C | 2553.5 | Semi standard non polar | 33892256 |
| Pantetheine,3TMS,isomer #10 | CC(C)(CO)C(O)C(=O)N(CCC(=O)N(CCS[Si](C)(C)C)[Si](C)(C)C)[Si](C)(C)C | 2626.8 | Standard non polar | 33892256 |
| Pantetheine,3TMS,isomer #10 | CC(C)(CO)C(O)C(=O)N(CCC(=O)N(CCS[Si](C)(C)C)[Si](C)(C)C)[Si](C)(C)C | 3189.5 | Standard polar | 33892256 |
| Pantetheine,3TMS,isomer #2 | CC(C)(CO[Si](C)(C)C)C(O[Si](C)(C)C)C(=O)N(CCC(=O)NCCS)[Si](C)(C)C | 2494.5 | Semi standard non polar | 33892256 |
| Pantetheine,3TMS,isomer #2 | CC(C)(CO[Si](C)(C)C)C(O[Si](C)(C)C)C(=O)N(CCC(=O)NCCS)[Si](C)(C)C | 2393.1 | Standard non polar | 33892256 |
| Pantetheine,3TMS,isomer #2 | CC(C)(CO[Si](C)(C)C)C(O[Si](C)(C)C)C(=O)N(CCC(=O)NCCS)[Si](C)(C)C | 2791.6 | Standard polar | 33892256 |
| Pantetheine,3TMS,isomer #3 | CC(C)(CO[Si](C)(C)C)C(O[Si](C)(C)C)C(=O)NCCC(=O)N(CCS)[Si](C)(C)C | 2457.5 | Semi standard non polar | 33892256 |
| Pantetheine,3TMS,isomer #3 | CC(C)(CO[Si](C)(C)C)C(O[Si](C)(C)C)C(=O)NCCC(=O)N(CCS)[Si](C)(C)C | 2446.2 | Standard non polar | 33892256 |
| Pantetheine,3TMS,isomer #3 | CC(C)(CO[Si](C)(C)C)C(O[Si](C)(C)C)C(=O)NCCC(=O)N(CCS)[Si](C)(C)C | 2774.8 | Standard polar | 33892256 |
| Pantetheine,3TMS,isomer #4 | CC(C)(CO[Si](C)(C)C)C(O)C(=O)N(CCC(=O)NCCS[Si](C)(C)C)[Si](C)(C)C | 2574.4 | Semi standard non polar | 33892256 |
| Pantetheine,3TMS,isomer #4 | CC(C)(CO[Si](C)(C)C)C(O)C(=O)N(CCC(=O)NCCS[Si](C)(C)C)[Si](C)(C)C | 2576.8 | Standard non polar | 33892256 |
| Pantetheine,3TMS,isomer #4 | CC(C)(CO[Si](C)(C)C)C(O)C(=O)N(CCC(=O)NCCS[Si](C)(C)C)[Si](C)(C)C | 3033.7 | Standard polar | 33892256 |
| Pantetheine,3TMS,isomer #5 | CC(C)(CO[Si](C)(C)C)C(O)C(=O)NCCC(=O)N(CCS[Si](C)(C)C)[Si](C)(C)C | 2617.8 | Semi standard non polar | 33892256 |
| Pantetheine,3TMS,isomer #5 | CC(C)(CO[Si](C)(C)C)C(O)C(=O)NCCC(=O)N(CCS[Si](C)(C)C)[Si](C)(C)C | 2610.2 | Standard non polar | 33892256 |
| Pantetheine,3TMS,isomer #5 | CC(C)(CO[Si](C)(C)C)C(O)C(=O)NCCC(=O)N(CCS[Si](C)(C)C)[Si](C)(C)C | 3046.7 | Standard polar | 33892256 |
| Pantetheine,3TMS,isomer #6 | CC(C)(CO[Si](C)(C)C)C(O)C(=O)N(CCC(=O)N(CCS)[Si](C)(C)C)[Si](C)(C)C | 2453.1 | Semi standard non polar | 33892256 |
| Pantetheine,3TMS,isomer #6 | CC(C)(CO[Si](C)(C)C)C(O)C(=O)N(CCC(=O)N(CCS)[Si](C)(C)C)[Si](C)(C)C | 2526.0 | Standard non polar | 33892256 |
| Pantetheine,3TMS,isomer #6 | CC(C)(CO[Si](C)(C)C)C(O)C(=O)N(CCC(=O)N(CCS)[Si](C)(C)C)[Si](C)(C)C | 2874.7 | Standard polar | 33892256 |
| Pantetheine,3TMS,isomer #7 | CC(C)(CO)C(O[Si](C)(C)C)C(=O)N(CCC(=O)NCCS[Si](C)(C)C)[Si](C)(C)C | 2538.5 | Semi standard non polar | 33892256 |
| Pantetheine,3TMS,isomer #7 | CC(C)(CO)C(O[Si](C)(C)C)C(=O)N(CCC(=O)NCCS[Si](C)(C)C)[Si](C)(C)C | 2529.7 | Standard non polar | 33892256 |
| Pantetheine,3TMS,isomer #7 | CC(C)(CO)C(O[Si](C)(C)C)C(=O)N(CCC(=O)NCCS[Si](C)(C)C)[Si](C)(C)C | 3014.3 | Standard polar | 33892256 |
| Pantetheine,3TMS,isomer #8 | CC(C)(CO)C(O[Si](C)(C)C)C(=O)NCCC(=O)N(CCS[Si](C)(C)C)[Si](C)(C)C | 2538.9 | Semi standard non polar | 33892256 |
| Pantetheine,3TMS,isomer #8 | CC(C)(CO)C(O[Si](C)(C)C)C(=O)NCCC(=O)N(CCS[Si](C)(C)C)[Si](C)(C)C | 2550.3 | Standard non polar | 33892256 |
| Pantetheine,3TMS,isomer #8 | CC(C)(CO)C(O[Si](C)(C)C)C(=O)NCCC(=O)N(CCS[Si](C)(C)C)[Si](C)(C)C | 3018.7 | Standard polar | 33892256 |
| Pantetheine,3TMS,isomer #9 | CC(C)(CO)C(O[Si](C)(C)C)C(=O)N(CCC(=O)N(CCS)[Si](C)(C)C)[Si](C)(C)C | 2428.4 | Semi standard non polar | 33892256 |
| Pantetheine,3TMS,isomer #9 | CC(C)(CO)C(O[Si](C)(C)C)C(=O)N(CCC(=O)N(CCS)[Si](C)(C)C)[Si](C)(C)C | 2461.8 | Standard non polar | 33892256 |
| Pantetheine,3TMS,isomer #9 | CC(C)(CO)C(O[Si](C)(C)C)C(=O)N(CCC(=O)N(CCS)[Si](C)(C)C)[Si](C)(C)C | 2863.6 | Standard polar | 33892256 |
| Pantetheine,4TMS,isomer #1 | CC(C)(CO[Si](C)(C)C)C(O[Si](C)(C)C)C(=O)N(CCC(=O)NCCS[Si](C)(C)C)[Si](C)(C)C | 2535.8 | Semi standard non polar | 33892256 |
| Pantetheine,4TMS,isomer #1 | CC(C)(CO[Si](C)(C)C)C(O[Si](C)(C)C)C(=O)N(CCC(=O)NCCS[Si](C)(C)C)[Si](C)(C)C | 2558.4 | Standard non polar | 33892256 |
| Pantetheine,4TMS,isomer #1 | CC(C)(CO[Si](C)(C)C)C(O[Si](C)(C)C)C(=O)N(CCC(=O)NCCS[Si](C)(C)C)[Si](C)(C)C | 2597.9 | Standard polar | 33892256 |
| Pantetheine,4TMS,isomer #2 | CC(C)(CO[Si](C)(C)C)C(O[Si](C)(C)C)C(=O)NCCC(=O)N(CCS[Si](C)(C)C)[Si](C)(C)C | 2558.0 | Semi standard non polar | 33892256 |
| Pantetheine,4TMS,isomer #2 | CC(C)(CO[Si](C)(C)C)C(O[Si](C)(C)C)C(=O)NCCC(=O)N(CCS[Si](C)(C)C)[Si](C)(C)C | 2572.6 | Standard non polar | 33892256 |
| Pantetheine,4TMS,isomer #2 | CC(C)(CO[Si](C)(C)C)C(O[Si](C)(C)C)C(=O)NCCC(=O)N(CCS[Si](C)(C)C)[Si](C)(C)C | 2605.2 | Standard polar | 33892256 |
| Pantetheine,4TMS,isomer #3 | CC(C)(CO[Si](C)(C)C)C(O[Si](C)(C)C)C(=O)N(CCC(=O)N(CCS)[Si](C)(C)C)[Si](C)(C)C | 2447.1 | Semi standard non polar | 33892256 |
| Pantetheine,4TMS,isomer #3 | CC(C)(CO[Si](C)(C)C)C(O[Si](C)(C)C)C(=O)N(CCC(=O)N(CCS)[Si](C)(C)C)[Si](C)(C)C | 2546.2 | Standard non polar | 33892256 |
| Pantetheine,4TMS,isomer #3 | CC(C)(CO[Si](C)(C)C)C(O[Si](C)(C)C)C(=O)N(CCC(=O)N(CCS)[Si](C)(C)C)[Si](C)(C)C | 2700.7 | Standard polar | 33892256 |
| Pantetheine,4TMS,isomer #4 | CC(C)(CO[Si](C)(C)C)C(O)C(=O)N(CCC(=O)N(CCS[Si](C)(C)C)[Si](C)(C)C)[Si](C)(C)C | 2568.0 | Semi standard non polar | 33892256 |
| Pantetheine,4TMS,isomer #4 | CC(C)(CO[Si](C)(C)C)C(O)C(=O)N(CCC(=O)N(CCS[Si](C)(C)C)[Si](C)(C)C)[Si](C)(C)C | 2651.9 | Standard non polar | 33892256 |
| Pantetheine,4TMS,isomer #4 | CC(C)(CO[Si](C)(C)C)C(O)C(=O)N(CCC(=O)N(CCS[Si](C)(C)C)[Si](C)(C)C)[Si](C)(C)C | 2866.5 | Standard polar | 33892256 |
| Pantetheine,4TMS,isomer #5 | CC(C)(CO)C(O[Si](C)(C)C)C(=O)N(CCC(=O)N(CCS[Si](C)(C)C)[Si](C)(C)C)[Si](C)(C)C | 2539.7 | Semi standard non polar | 33892256 |
| Pantetheine,4TMS,isomer #5 | CC(C)(CO)C(O[Si](C)(C)C)C(=O)N(CCC(=O)N(CCS[Si](C)(C)C)[Si](C)(C)C)[Si](C)(C)C | 2611.0 | Standard non polar | 33892256 |
| Pantetheine,4TMS,isomer #5 | CC(C)(CO)C(O[Si](C)(C)C)C(=O)N(CCC(=O)N(CCS[Si](C)(C)C)[Si](C)(C)C)[Si](C)(C)C | 2845.5 | Standard polar | 33892256 |
| Pantetheine,5TMS,isomer #1 | CC(C)(CO[Si](C)(C)C)C(O[Si](C)(C)C)C(=O)N(CCC(=O)N(CCS[Si](C)(C)C)[Si](C)(C)C)[Si](C)(C)C | 2579.7 | Semi standard non polar | 33892256 |
| Pantetheine,5TMS,isomer #1 | CC(C)(CO[Si](C)(C)C)C(O[Si](C)(C)C)C(=O)N(CCC(=O)N(CCS[Si](C)(C)C)[Si](C)(C)C)[Si](C)(C)C | 2646.5 | Standard non polar | 33892256 |
| Pantetheine,5TMS,isomer #1 | CC(C)(CO[Si](C)(C)C)C(O[Si](C)(C)C)C(=O)N(CCC(=O)N(CCS[Si](C)(C)C)[Si](C)(C)C)[Si](C)(C)C | 2474.9 | Standard polar | 33892256 |
| Pantetheine,1TBDMS,isomer #1 | CC(C)(CO[Si](C)(C)C(C)(C)C)C(O)C(=O)NCCC(=O)NCCS | 2789.4 | Semi standard non polar | 33892256 |
| Pantetheine,1TBDMS,isomer #2 | CC(C)(CO)C(O[Si](C)(C)C(C)(C)C)C(=O)NCCC(=O)NCCS | 2737.0 | Semi standard non polar | 33892256 |
| Pantetheine,1TBDMS,isomer #3 | CC(C)(CO)C(O)C(=O)NCCC(=O)NCCS[Si](C)(C)C(C)(C)C | 2818.2 | Semi standard non polar | 33892256 |
| Pantetheine,1TBDMS,isomer #4 | CC(C)(CO)C(O)C(=O)N(CCC(=O)NCCS)[Si](C)(C)C(C)(C)C | 2715.9 | Semi standard non polar | 33892256 |
| Pantetheine,1TBDMS,isomer #5 | CC(C)(CO)C(O)C(=O)NCCC(=O)N(CCS)[Si](C)(C)C(C)(C)C | 2736.2 | Semi standard non polar | 33892256 |
| Pantetheine,2TBDMS,isomer #1 | CC(C)(CO[Si](C)(C)C(C)(C)C)C(O[Si](C)(C)C(C)(C)C)C(=O)NCCC(=O)NCCS | 2996.8 | Semi standard non polar | 33892256 |
| Pantetheine,2TBDMS,isomer #10 | CC(C)(CO)C(O)C(=O)N(CCC(=O)N(CCS)[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2931.8 | Semi standard non polar | 33892256 |
| Pantetheine,2TBDMS,isomer #2 | CC(C)(CO[Si](C)(C)C(C)(C)C)C(O)C(=O)NCCC(=O)NCCS[Si](C)(C)C(C)(C)C | 3111.6 | Semi standard non polar | 33892256 |
| Pantetheine,2TBDMS,isomer #3 | CC(C)(CO[Si](C)(C)C(C)(C)C)C(O)C(=O)N(CCC(=O)NCCS)[Si](C)(C)C(C)(C)C | 2988.3 | Semi standard non polar | 33892256 |
| Pantetheine,2TBDMS,isomer #4 | CC(C)(CO[Si](C)(C)C(C)(C)C)C(O)C(=O)NCCC(=O)N(CCS)[Si](C)(C)C(C)(C)C | 3007.6 | Semi standard non polar | 33892256 |
| Pantetheine,2TBDMS,isomer #5 | CC(C)(CO)C(O[Si](C)(C)C(C)(C)C)C(=O)NCCC(=O)NCCS[Si](C)(C)C(C)(C)C | 3029.6 | Semi standard non polar | 33892256 |
| Pantetheine,2TBDMS,isomer #6 | CC(C)(CO)C(O[Si](C)(C)C(C)(C)C)C(=O)N(CCC(=O)NCCS)[Si](C)(C)C(C)(C)C | 2952.6 | Semi standard non polar | 33892256 |
| Pantetheine,2TBDMS,isomer #7 | CC(C)(CO)C(O[Si](C)(C)C(C)(C)C)C(=O)NCCC(=O)N(CCS)[Si](C)(C)C(C)(C)C | 2925.7 | Semi standard non polar | 33892256 |
| Pantetheine,2TBDMS,isomer #8 | CC(C)(CO)C(O)C(=O)N(CCC(=O)NCCS[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 3064.4 | Semi standard non polar | 33892256 |
| Pantetheine,2TBDMS,isomer #9 | CC(C)(CO)C(O)C(=O)NCCC(=O)N(CCS[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 3137.9 | Semi standard non polar | 33892256 |
| Pantetheine,3TBDMS,isomer #1 | CC(C)(CO[Si](C)(C)C(C)(C)C)C(O[Si](C)(C)C(C)(C)C)C(=O)NCCC(=O)NCCS[Si](C)(C)C(C)(C)C | 3264.6 | Semi standard non polar | 33892256 |
| Pantetheine,3TBDMS,isomer #1 | CC(C)(CO[Si](C)(C)C(C)(C)C)C(O[Si](C)(C)C(C)(C)C)C(=O)NCCC(=O)NCCS[Si](C)(C)C(C)(C)C | 3098.7 | Standard non polar | 33892256 |
| Pantetheine,3TBDMS,isomer #1 | CC(C)(CO[Si](C)(C)C(C)(C)C)C(O[Si](C)(C)C(C)(C)C)C(=O)NCCC(=O)NCCS[Si](C)(C)C(C)(C)C | 3041.4 | Standard polar | 33892256 |
| Pantetheine,3TBDMS,isomer #10 | CC(C)(CO)C(O)C(=O)N(CCC(=O)N(CCS[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 3288.0 | Semi standard non polar | 33892256 |
| Pantetheine,3TBDMS,isomer #10 | CC(C)(CO)C(O)C(=O)N(CCC(=O)N(CCS[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 3173.9 | Standard non polar | 33892256 |
| Pantetheine,3TBDMS,isomer #10 | CC(C)(CO)C(O)C(=O)N(CCC(=O)N(CCS[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 3313.3 | Standard polar | 33892256 |
| Pantetheine,3TBDMS,isomer #2 | CC(C)(CO[Si](C)(C)C(C)(C)C)C(O[Si](C)(C)C(C)(C)C)C(=O)N(CCC(=O)NCCS)[Si](C)(C)C(C)(C)C | 3174.7 | Semi standard non polar | 33892256 |
| Pantetheine,3TBDMS,isomer #2 | CC(C)(CO[Si](C)(C)C(C)(C)C)C(O[Si](C)(C)C(C)(C)C)C(=O)N(CCC(=O)NCCS)[Si](C)(C)C(C)(C)C | 3007.9 | Standard non polar | 33892256 |
| Pantetheine,3TBDMS,isomer #2 | CC(C)(CO[Si](C)(C)C(C)(C)C)C(O[Si](C)(C)C(C)(C)C)C(=O)N(CCC(=O)NCCS)[Si](C)(C)C(C)(C)C | 3056.6 | Standard polar | 33892256 |
| Pantetheine,3TBDMS,isomer #3 | CC(C)(CO[Si](C)(C)C(C)(C)C)C(O[Si](C)(C)C(C)(C)C)C(=O)NCCC(=O)N(CCS)[Si](C)(C)C(C)(C)C | 3150.4 | Semi standard non polar | 33892256 |
| Pantetheine,3TBDMS,isomer #3 | CC(C)(CO[Si](C)(C)C(C)(C)C)C(O[Si](C)(C)C(C)(C)C)C(=O)NCCC(=O)N(CCS)[Si](C)(C)C(C)(C)C | 3026.9 | Standard non polar | 33892256 |
| Pantetheine,3TBDMS,isomer #3 | CC(C)(CO[Si](C)(C)C(C)(C)C)C(O[Si](C)(C)C(C)(C)C)C(=O)NCCC(=O)N(CCS)[Si](C)(C)C(C)(C)C | 3038.6 | Standard polar | 33892256 |
| Pantetheine,3TBDMS,isomer #4 | CC(C)(CO[Si](C)(C)C(C)(C)C)C(O)C(=O)N(CCC(=O)NCCS[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 3294.5 | Semi standard non polar | 33892256 |
| Pantetheine,3TBDMS,isomer #4 | CC(C)(CO[Si](C)(C)C(C)(C)C)C(O)C(=O)N(CCC(=O)NCCS[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 3161.5 | Standard non polar | 33892256 |
| Pantetheine,3TBDMS,isomer #4 | CC(C)(CO[Si](C)(C)C(C)(C)C)C(O)C(=O)N(CCC(=O)NCCS[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 3202.6 | Standard polar | 33892256 |
| Pantetheine,3TBDMS,isomer #5 | CC(C)(CO[Si](C)(C)C(C)(C)C)C(O)C(=O)NCCC(=O)N(CCS[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 3379.1 | Semi standard non polar | 33892256 |
| Pantetheine,3TBDMS,isomer #5 | CC(C)(CO[Si](C)(C)C(C)(C)C)C(O)C(=O)NCCC(=O)N(CCS[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 3170.7 | Standard non polar | 33892256 |
| Pantetheine,3TBDMS,isomer #5 | CC(C)(CO[Si](C)(C)C(C)(C)C)C(O)C(=O)NCCC(=O)N(CCS[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 3216.9 | Standard polar | 33892256 |
| Pantetheine,3TBDMS,isomer #6 | CC(C)(CO[Si](C)(C)C(C)(C)C)C(O)C(=O)N(CCC(=O)N(CCS)[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 3179.9 | Semi standard non polar | 33892256 |
| Pantetheine,3TBDMS,isomer #6 | CC(C)(CO[Si](C)(C)C(C)(C)C)C(O)C(=O)N(CCC(=O)N(CCS)[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 3086.5 | Standard non polar | 33892256 |
| Pantetheine,3TBDMS,isomer #6 | CC(C)(CO[Si](C)(C)C(C)(C)C)C(O)C(=O)N(CCC(=O)N(CCS)[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 3131.3 | Standard polar | 33892256 |
| Pantetheine,3TBDMS,isomer #7 | CC(C)(CO)C(O[Si](C)(C)C(C)(C)C)C(=O)N(CCC(=O)NCCS[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 3241.1 | Semi standard non polar | 33892256 |
| Pantetheine,3TBDMS,isomer #7 | CC(C)(CO)C(O[Si](C)(C)C(C)(C)C)C(=O)N(CCC(=O)NCCS[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 3128.4 | Standard non polar | 33892256 |
| Pantetheine,3TBDMS,isomer #7 | CC(C)(CO)C(O[Si](C)(C)C(C)(C)C)C(=O)N(CCC(=O)NCCS[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 3183.6 | Standard polar | 33892256 |
| Pantetheine,3TBDMS,isomer #8 | CC(C)(CO)C(O[Si](C)(C)C(C)(C)C)C(=O)NCCC(=O)N(CCS[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 3292.7 | Semi standard non polar | 33892256 |
| Pantetheine,3TBDMS,isomer #8 | CC(C)(CO)C(O[Si](C)(C)C(C)(C)C)C(=O)NCCC(=O)N(CCS[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 3129.3 | Standard non polar | 33892256 |
| Pantetheine,3TBDMS,isomer #8 | CC(C)(CO)C(O[Si](C)(C)C(C)(C)C)C(=O)NCCC(=O)N(CCS[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 3189.4 | Standard polar | 33892256 |
| Pantetheine,3TBDMS,isomer #9 | CC(C)(CO)C(O[Si](C)(C)C(C)(C)C)C(=O)N(CCC(=O)N(CCS)[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 3153.1 | Semi standard non polar | 33892256 |
| Pantetheine,3TBDMS,isomer #9 | CC(C)(CO)C(O[Si](C)(C)C(C)(C)C)C(=O)N(CCC(=O)N(CCS)[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 3053.5 | Standard non polar | 33892256 |
| Pantetheine,3TBDMS,isomer #9 | CC(C)(CO)C(O[Si](C)(C)C(C)(C)C)C(=O)N(CCC(=O)N(CCS)[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 3115.0 | Standard polar | 33892256 |
| Pantetheine,4TBDMS,isomer #1 | CC(C)(CO[Si](C)(C)C(C)(C)C)C(O[Si](C)(C)C(C)(C)C)C(=O)N(CCC(=O)NCCS[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 3455.2 | Semi standard non polar | 33892256 |
| Pantetheine,4TBDMS,isomer #1 | CC(C)(CO[Si](C)(C)C(C)(C)C)C(O[Si](C)(C)C(C)(C)C)C(=O)N(CCC(=O)NCCS[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 3289.8 | Standard non polar | 33892256 |
| Pantetheine,4TBDMS,isomer #1 | CC(C)(CO[Si](C)(C)C(C)(C)C)C(O[Si](C)(C)C(C)(C)C)C(=O)N(CCC(=O)NCCS[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2980.4 | Standard polar | 33892256 |
| Pantetheine,4TBDMS,isomer #2 | CC(C)(CO[Si](C)(C)C(C)(C)C)C(O[Si](C)(C)C(C)(C)C)C(=O)NCCC(=O)N(CCS[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 3507.1 | Semi standard non polar | 33892256 |
| Pantetheine,4TBDMS,isomer #2 | CC(C)(CO[Si](C)(C)C(C)(C)C)C(O[Si](C)(C)C(C)(C)C)C(=O)NCCC(=O)N(CCS[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 3276.2 | Standard non polar | 33892256 |
| Pantetheine,4TBDMS,isomer #2 | CC(C)(CO[Si](C)(C)C(C)(C)C)C(O[Si](C)(C)C(C)(C)C)C(=O)NCCC(=O)N(CCS[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2992.3 | Standard polar | 33892256 |
| Pantetheine,4TBDMS,isomer #3 | CC(C)(CO[Si](C)(C)C(C)(C)C)C(O[Si](C)(C)C(C)(C)C)C(=O)N(CCC(=O)N(CCS)[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 3364.4 | Semi standard non polar | 33892256 |
| Pantetheine,4TBDMS,isomer #3 | CC(C)(CO[Si](C)(C)C(C)(C)C)C(O[Si](C)(C)C(C)(C)C)C(=O)N(CCC(=O)N(CCS)[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 3289.8 | Standard non polar | 33892256 |
| Pantetheine,4TBDMS,isomer #3 | CC(C)(CO[Si](C)(C)C(C)(C)C)C(O[Si](C)(C)C(C)(C)C)C(=O)N(CCC(=O)N(CCS)[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 3075.4 | Standard polar | 33892256 |
| Pantetheine,4TBDMS,isomer #4 | CC(C)(CO[Si](C)(C)C(C)(C)C)C(O)C(=O)N(CCC(=O)N(CCS[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 3509.5 | Semi standard non polar | 33892256 |
| Pantetheine,4TBDMS,isomer #4 | CC(C)(CO[Si](C)(C)C(C)(C)C)C(O)C(=O)N(CCC(=O)N(CCS[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 3343.3 | Standard non polar | 33892256 |
| Pantetheine,4TBDMS,isomer #4 | CC(C)(CO[Si](C)(C)C(C)(C)C)C(O)C(=O)N(CCC(=O)N(CCS[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 3165.7 | Standard polar | 33892256 |
| Pantetheine,4TBDMS,isomer #5 | CC(C)(CO)C(O[Si](C)(C)C(C)(C)C)C(=O)N(CCC(=O)N(CCS[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 3483.7 | Semi standard non polar | 33892256 |
| Pantetheine,4TBDMS,isomer #5 | CC(C)(CO)C(O[Si](C)(C)C(C)(C)C)C(=O)N(CCC(=O)N(CCS[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 3319.1 | Standard non polar | 33892256 |
| Pantetheine,4TBDMS,isomer #5 | CC(C)(CO)C(O[Si](C)(C)C(C)(C)C)C(=O)N(CCC(=O)N(CCS[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 3143.1 | Standard polar | 33892256 |
| Pantetheine,5TBDMS,isomer #1 | CC(C)(CO[Si](C)(C)C(C)(C)C)C(O[Si](C)(C)C(C)(C)C)C(=O)N(CCC(=O)N(CCS[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 3699.5 | Semi standard non polar | 33892256 |
| Pantetheine,5TBDMS,isomer #1 | CC(C)(CO[Si](C)(C)C(C)(C)C)C(O[Si](C)(C)C(C)(C)C)C(=O)N(CCC(=O)N(CCS[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 3475.0 | Standard non polar | 33892256 |
| Pantetheine,5TBDMS,isomer #1 | CC(C)(CO[Si](C)(C)C(C)(C)C)C(O[Si](C)(C)C(C)(C)C)C(=O)N(CCC(=O)N(CCS[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2997.7 | Standard polar | 33892256 |